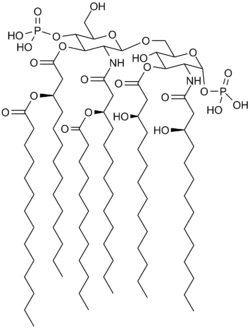
Eritoran
Eritoran is an investigational drug for the treatment of severe sepsis, an excessive inflammatory response to an infection.
 | |
| Clinical data | |
|---|---|
| Other names | E 5564 |
| Routes of administration | Intravenous injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C66H126N2O19P2 |
| Molar mass | 1313.656 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
In a phase III clinical trial,[1] eritoran did not perform better than existing treatments for the treatment of sepsis.[2][3]
It was being developed by the Japanese pharmaceutical company Eisai Co. and administered intravenously as the sodium salt eritoran tetrasodium.[4][5]
Mechanism of action
Toll-like receptors (TLRs) play an important role in the innate immune system. They recognise microbes and activate inflammatory immune responses. Toll-like receptor 4 (TLR4) detects lipopolysaccharides found in most Gram-negative bacteria.[6]
Because of its similarity to the lipopolysaccharide lipid A, eritoran acts as TLR4 antagonist and so blocks the excessive reaction triggered by this receptor.[5][7]
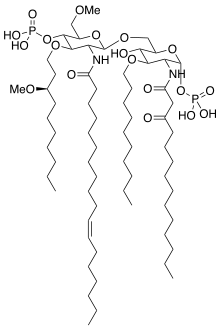 Eritoran |
Cytokine storm
While eritoran did not perform well in the treatment of sepsis, it was shown to combat another, related phenomenon called cytokine storm in influenza cases involving certain virus strains (involving preliminary experimentation on mice, not in other animals or humans, led by a University of Maryland School of Medicine researcher).A further study in mice and rats by the same group[8] showed it prevented acute lung injury. A cytokine storm can help to cause sepsis and can in concert with it or by itself cause serious illness or death if not soon controlled. Mortality rates for sepsis, cytokine storm, and especially septic shock and organ dysfunction are still quite high despite progress made. This is in no small part due to the prevalence of nosocomial (hospital-acquired) infections, as well as ongoing mutations which confer multi-drug resistance in pathological microorganisms such as bacteria and viruses (most strains of flu are resistant to amantadine and rimantadine, and some are resistant to oseltamivir), and delays and mistakes in the recognition and treatment of disease.[9] New flu strains, such as the H7N9 strain, are always emerging.
Eritoran, because of its structural similarity to the gram-negative bacterial lipopolysaccharide (lipid A) acts as TLR4 antagonist. Eritoran didn't perform well in phase III clinical trials, however it successfully treated cytokine storm in influenza animal models.[8] There were multiple factors that could be attributed to the failure of Eritoran against sepsis, which include poorly designed lipid A scaffold, antagonist designed using mice model where as it is known that there exists species differences (human vs mice) in lipid A recognition, role of MD2/TLR4 PTMs on receptor function is not fully understood, recruitment of heterogeneous patient population, and lack of a well-defined structure activity relationship (SAR) of LPS interaction with MD2(TLR4).[10]
References
- Clinical trial number NCT00334828 for "ACCESS: A Controlled Comparison of Eritoran Tetrasodium and Placebo in Patients With Severe Sepsis" at ClinicalTrials.gov
- Steven M. Opal; et al. (2013). "Effect of Eritoran, an Antagonist of MD2-TLR4, on Mortality in Patients With Severe Sepsis: The ACCESS Randomized Trial". JAMA. 309 (11): 1154–1162. doi:10.1001/jama.2013.2194. hdl:1854/LU-4222072. PMID 23512062.
- "Phase III Study for Eritoran Does Not Meet Primary Endpoint". drugs.com.
- "Eritoran: A Potential Therapeutic Agent In Severe Sepsis". MediNEWS.Direct. 17 October 2007. Archived from the original on 14 July 2011. Retrieved 26 December 2009.
- Kiemer, Alexandra K. (2008). "TLR eröffnen neue Möglichkeiten". Pharmazeutische Zeitung online (in German). Govi-Verlag. Retrieved 26 December 2009.
- "Entrez Gene: TLR4 toll-like receptor 4".
- Tidswell, M; Tillis, W; Larosa, SP; Lynn, M; Wittek, AE; Kao, R; Wheeler, J; Gogate, J; et al. (2010). "Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis". Critical Care Medicine. 38 (1): 72–83. doi:10.1097/CCM.0b013e3181b07b78. PMID 19661804.
- Shirey, K A; Lai, W; Scott, A J; et al. (2013). "TLR4 antagonist Eritoran protects mice from lethal influenza infection". Nature. 497 (7450): 498–503. doi:10.1038/nature12118. PMC 3725830. PMID 23636320.
- "New drug offers novel approach to taming flu virus". NBC News. 1 May 2013.
- Fink, Mitchell P.; Warren, H. Shaw (2014). "Strategies to improve drug development for sepsis". Nature Reviews Drug Discovery. 13 (10): 741–758. doi:10.1038/nrd4368. PMID 25190187.