Entamoeba histolytica
Entamoeba histolytica is an anaerobic parasitic amoebozoan, part of the genus Entamoeba.[1] Predominantly infecting humans and other primates causing amoebiasis, E. histolytica is estimated to infect about 50 million people worldwide. E. histolytica infection is estimated to kill more than 55,000 people each year.[2] Previously, it was thought that 10% of the world population was infected, but these figures predate the recognition that at least 90% of these infections were due to a second species, E. dispar.[3] Mammals such as dogs and cats can become infected transiently, but are not thought to contribute significantly to transmission.
| Entamoeba histolytica | |
|---|---|
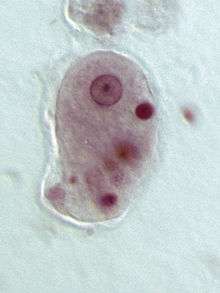 | |
| Entamoeba histolytica trophozoite | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | |
| Subphylum: | |
| Class: | |
| Family: | |
| Genus: | |
| Species: | E. histolytica |
| Binomial name | |
| Entamoeba histolytica Schaudinn, 1903 | |
The word histolysis literally means disintegration and dissolution of organic tissues.
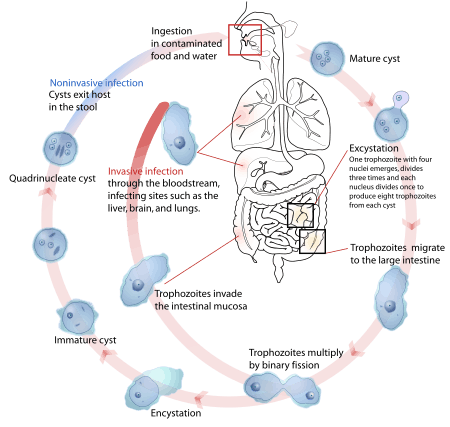
Transmission
The active (trophozoite) stage exists only in the host and in fresh loose feces; cysts survive outside the host in water, in soils, and on foods, especially under moist conditions on the latter. The infection can occur when a person puts anything into their mouth that has touched the feces of a person who is infected with E. histolytica, swallows something, such as water or food, that is contaminated with E. histolytica, or swallows E. histolytica cysts (eggs) picked up from contaminated surfaces or fingers.[4] The cysts are readily killed by heat and by freezing temperatures, and survive for only a few months outside of the host.[5] When cysts are swallowed they cause infections by excysting (releasing the trophozoite stage) in the digestive tract. The pathogenic nature of E. histolytica was first reported by Fyodor Lesh in 1875, but it was not given its Latin name until Fritz Schaudinn described it in 1903. E. histolytica, as its name suggests (histo–lytic = tissue destroying), is pathogenic; infection can be asymptomatic or can lead to amoebic dysentery or amoebic liver abscess.[1][6] Symptoms can include fulminating dysentery, bloody diarrhea, weight loss, fatigue, abdominal pain, and amoeboma. The amoeba can actually 'bore' into the intestinal wall, causing lesions and intestinal symptoms, and it may reach the blood stream. From there, it can reach different vital organs of the human body, usually the liver, but sometimes the lungs, brain, spleen, etc. A common outcome of this invasion of tissues is a liver abscess, which can be fatal if untreated. Ingested red blood cells are sometimes seen in the amoeba cell cytoplasm.
Risk factors
Poor sanitary conditions are known to increase the risk of contracting amebiasis E. histolytica.[7] In the United States, there is a much higher rate of amebiasis-related mortality in California and Texas, which might be caused by the proximity of those states to E. histolytica-endemic areas, such as Mexico, other parts of Latin America, and Asia.[8] E. histolytica is also recognized as an emerging sexually transmissible pathogen, especially in male homosexual relations, causing outbreaks in non-endemic regions.[9] As such, high-risk sex behaviour is also a potential source of infection.[10] Although it is unclear whether there is a causal link, studies indicate a higher chance of being infected with E. histolytica if one is also infected with HIV.[11][12]
Genome
The E. histolytica genome was sequenced, assembled, and automatically annotated in 2005.[13] The genome was reassembled and reannotated in 2010.[14] The 20 million basepair genome assembly contains 8,160 predicted genes; known and novel transposable elements have been mapped and characterized, functional assignments have been revised and updated, and additional information has been incorporated, including metabolic pathways, Gene Ontology assignments, curation of transporters, and generation of gene families.[15] The major group of transposable elements in E. histolytica are non-LTR retrotransposons. These have been divided in three families called EhLINEs and EhSINEs (EhLINE1,2,3 and EhSINE1,2,3).[16] EhLINE1 encode an endonuclease (EN) protein (in addition to reverse transcriptase and nucleotide-binding ORF1), which have similarity with bacterial restriction endonuclease. This similarity with bacterial protein indicates that transposable elements have been acquired from prokaryotes by horizontal gene transfer in this protozoan parasite.[17]
Pathology
In the vast majority of cases, infection is asymptomatic and the carrier is unaware they are infected. However, in an estimated 10% of cases E. histolytica causes disease. Once the trophozoites are excysted they colonize the large bowel, remaining on the surface of the mucus layer and feeding on bacteria and food particles. Occasionally, and in response to unknown stimuli, trophozoites move through the mucus layer where they come in contact with the epithelial cell layer and start the pathological process. E. histolytica has a lectin that binds to galactose and N-acetylgalactosamine sugars on the surface of the epithelial cells, The lectin normally is used to bind bacteria for ingestion. The parasite has several enzymes such as pore forming proteins, lipases, and cysteine proteases, which are normally used to digest bacteria in food vacuoles but which can cause lysis of the epithelial cells by inducing cellular necrosis and apoptosis when the trophozoite comes in contact with them and binds via the lectin. Enzymes released allow penetration into intestinal wall and blood vessels, sometimes on to liver and other organs.[18] The trophozoites will then ingest these dead cells. This damage to the epithelial cell layer attracts human immune cells and these in turn can be lysed by the trophozoite, which releases the immune cell's own lytic enzymes into the surrounding tissue, creating a type of chain reaction and leading to tissue destruction. This destruction manifests itself in the form of an 'ulcer' in the tissue, typically described as flask-shaped because of its appearance in transverse section. This tissue destruction can also involve blood vessels leading to bloody diarrhea, amebic dysentery. Occasionally, trophozoites enter the bloodstream where they are transported typically to the liver via the portal system. In the liver a similar pathological sequence ensues, leading to amebic liver abscesses. The trophozoites can also end up in other organs, sometimes via the bloodstream, sometimes via liver abscess rupture or fistulas. In all locations, similar pathology can occur.
Pathogen interaction
E. histolytica may modulate the virulence of certain human viruses and is itself a host for its own viruses.
For example, AIDS accentuates the damage and pathogenicity of E. histolytica.[12] On the other hand, cells infected with HIV are often consumed by E. histolytica. Infective HIV remains viable within the amoeba, although there has been no proof of human reinfection from amoeba carrying this virus.[19]
A burst of research on viruses of E. histolytica stems from a series of papers published by Diamond et al. from 1972 to 1979. In 1972, they hypothesized two separate polyhedral and filamentous viral strains within E. histolytica that caused cell lysis. Perhaps the most novel observation was that two kinds of viral strains existed, and that within one type of amoeba (strain HB-301) the polyhedral strain had no detrimental effect but led to cell lysis in another (strain HK-9). Although Mattern et al. attempted to explore the possibility that these protozoal viruses could function like bacteriophages, they found no significant changes in Entamoeba histolytica virulence when infected by viruses.[20]
Pathogenesis
E. histolytica causes tissue destruction which leads to clinical disease. E. histolytica–induced tissue damage by three main events: direct host cell death, inflammation, and parasite invasion.[21]
Diagnosis
Diagnosis is confirmed by microscopic examination for trophozoites or cysts in fresh or suitably preserved faecal specimens, smears of aspirates or scrapings obtained by proctoscopy, and aspirates of abscesses or other tissue specimen. A blood test is also available but is only recommended when a healthcare provider believes the infection may have spread beyond the intestine (gut) to some other organ of the body, such as the liver. However, this blood test may not be helpful in diagnosing current illness because the test can be positive if the patient has had amebiasis in the past, even if they are not infected at present.[22] Stool antigen detection and PCR are available for diagnosis, and are more sensitive and specific than microscopy.[2]
 Amoebic intestinal ulcer caused by E. histolytica
Amoebic intestinal ulcer caused by E. histolytica- Trophozoites of E. histolytica with ingested erythrocytes
 E. histolytica cyst
E. histolytica cyst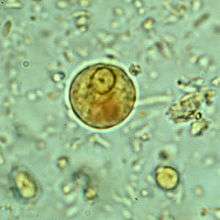 Immature E. histolytica cyst (mature cysts have 4 nuclei)
Immature E. histolytica cyst (mature cysts have 4 nuclei) Multiplication by binary fission
Multiplication by binary fission E. histolytica drawing
E. histolytica drawing_using_specific_anti%E2%80%93Entamoeba_histolytica_macrophage_migration_inhibitory_factor_antibodies_in_a_patient_with_amebic_colitis.jpg) Immunohistochemical staining of trophozoites (brown) using specific anti–Entamoeba histolytica macrophage migration inhibitory factor antibodies in a patient with amebic colitis
Immunohistochemical staining of trophozoites (brown) using specific anti–Entamoeba histolytica macrophage migration inhibitory factor antibodies in a patient with amebic colitis
Treatment
There are a number of effective medications. Generally several antibiotics are available to treat Entamoeba histolytica. The infected individual will be treated with only one antibiotic if the E. histolytica infection has not made the person sick and most likely be prescribed with two antibiotics if the person has been feeling sick.[23] Otherwise, below are other options for treatments.
Intestinal infection: Usually nitroimidazole derivatives (such as metronidazole) are used because they are highly effective against the trophozoite form of the amoeba. Since they have little effect on amoeba cysts, usually this treatment is followed by an agent (such as paromomycin or diloxanide furoate) that acts on the organism in the lumen.[2]
Liver abscess: In addition to targeting organisms in solid tissue, primarily with drugs like metronidazole and chloroquine, treatment of liver abscess must include agents that act in the lumen of the intestine (as in the preceding paragraph) to avoid re-invasion. Surgical drainage is usually not necessary except when rupture is imminent.[24]
People without symptoms: For people without symptoms (otherwise known as carriers, with no symptoms), non endemic areas should be treated by paromomycin, and other treatments include diloxanide furoate and iodoquinol. There have been problems with the use of iodoquinol and iodochlorhydroxyquin, so their use is not recommended. Diloxanide furoate can also be used by mildly symptomatic persons who are just passing cysts.
| Genus and species | Entamoeba histolytica |
| Etiologic agent of: | Amoebiasis; amoebic dysentery; extraintestinal amoebiasis, usually amoebic liver abscess; "anchovy sauce"); amoeba cutis; amoebic lung abscess ("liver-colored sputum") |
| Infective stage | Tetranucleated cyst (having 2-4 nuclei) |
| Definitive host | Human |
| Portal of entry | Mouth |
| Mode of transmission | Ingestion of mature cyst through contaminated food or water |
| Habitat | Colon and cecum |
| Pathogenic stage | Trophozoite |
| Locomotive apparatus | Pseudopodia ("false foot”") |
| Motility | Active, progressive and directional |
| Nucleus | 'Ring and dot' appearance: peripheral chromatin and central karyosome |
| Mode of reproduction | Binary fission |
| Pathogenesis | Lytic necrosis (it looks like “flask-shaped” holes in Gastrointestinal tract sections (GIT) |
| Type of encystment | Protective and Reproductive |
| Lab diagnosis | Most common is direct fecal smear (DFS) and staining (but does not allow identification to species level); enzyme immunoassay (EIA); indirect hemagglutination (IHA); Antigen detection – monoclonal antibody; PCR for species identification. Sometimes only the use of a fixative (formalin) is effective in detecting cysts. Culture: From faecal samples - Robinson's medium, Jones' medium |
| Treatment | Metronidazole for the invasive trophozoites PLUS a lumenal amoebicide for those still in the intestine. Paromomycin (Humatin) is the luminal drug of choice, since Diloxanide furoate (Furamide) is not commercially available in the United States or Canada (being available only from the Centers for Disease Control and Prevention). A direct comparison of efficacy showed that Paromomycin had a higher cure rate.[25] Paromomycin (Humatin) should be used with caution in patients with colitis, as it is both nephrotoxic and ototoxic. Absorption through the damaged wall of the intestinal tract can result in permanent hearing loss and kidney damage. Recommended dosage: metronidazole 750 mg three times a day orally, for 5 to 10 days followed by paromomycin 30 mg/kg/day orally in 3 equal doses for 5 to 10 days or Diloxanide furoate 500 mg 3 times a day orally for 10 days, to eradicate lumenal amoebae and prevent relapse.[26][27] |
| Trophozoite stage | |
| Pathognomonic/diagnostic feature | Ingested RBC; distinctive nucleus |
| Cyst Stage | |
| Chromatoidal body | 'Cigar' shaped bodies (made up of crystalline ribosomes) |
| Number of nuclei | 1 in early stages, 4 when mature |
| Pathognomonic/diagnostic feature | 'Ring and dot' nucleus and chromatoid bodies |
Meiosis
In sexually reproducing eukaryotes, homologous recombination (HR) ordinarily occurs during meiosis. The meiosis-specific recombinase, Dmc1, is required for efficient meiotic HR, and Dmc1 is expressed in E. histolytica.[28] The purified Dmc1 from E. histolytica forms presynaptic filaments and catalyzes ATP-dependent homologous DNA pairing and DNA strand exchange over at least several thousand base pairs.[28] The DNA pairing and strand exchange reactions are enhanced by the eukaryotic meiosis-specific recombination accessory factor (heterodimer) Hop2-Mnd1.[28] These processes are central to meiotic recombination, suggesting that E. histolytica undergoes meiosis.[28]
Several other genes involved in both mitotic and meiotic HR are also present in E. histolytica.[29] HR is enhanced under stressful growth conditions (serum starvation) concomitant with the up-regulation of HR-related genes.[30] Also, UV irradiation induces DNA damage in E. histolytica trophozoites and activates the recombinational DNA repair pathway.[29] In particular, expression of the Rad51 protein (a recombinase) is increased about 15-fold by UV treatment.
See also
References
- Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 733–8. ISBN 978-0-8385-8529-0.
- Shirley DT, Farr L, Watanabe K, Moonah S (July 2018). "A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis". Open Forum Infectious Diseases. 5 (7): ofy161. doi:10.1093/ofid/ofy161. PMC 6055529. PMID 30046644.
- "Amoebiasis" (PDF). Releve Epidemiologique Hebdomadaire. 72 (14): 97–9. April 1997. PMID 9100475.
- "Entamoeba histolytica". cdc.govPrevention. Center for Disease Control & Prevention. Retrieved 24 October 2017.
- American Water Works Association (June 2006). Waterborne Pathogens. American Water Works Association. ISBN 978-1-58321-403-9.
- Nespola B, Betz V, Brunet J, Gagnard JC, Krummel Y, Hansmann Y, et al. (2015). "First case of amebic liver abscess 22 years after the first occurrence". Parasite. 22: 20. doi:10.1051/parasite/2015020. PMC 4472968. PMID 26088504.

- "General Information | Amebiasis | Parasites | CDC". www.cdc.gov. Retrieved 2018-03-01.
- Gunther J, Shafir S, Bristow B, Sorvillo F (December 2011). "Short report: Amebiasis-related mortality among United States residents, 1990-2007". The American Journal of Tropical Medicine and Hygiene. 85 (6): 1038–40. doi:10.4269/ajtmh.2011.11-0288. PMC 3225148. PMID 22144440.
- Escolà-Vergé L, Arando M, Vall M, Rovira R, Espasa M, Sulleiro E, et al. (July 2017). "Outbreak of intestinal amoebiasis among men who have sex with men, Barcelona (Spain), October 2016 and January 2017". Euro Surveillance. 22 (30). doi:10.2807/1560-7917.ES.2017.22.30.30581. PMC 5553055. PMID 28797327.
- Stark D, van Hal SJ, Matthews G, Harkness J, Marriott D (July 2008). "Invasive amebiasis in men who have sex with men, Australia". Emerging Infectious Diseases. 14 (7): 1141–3. doi:10.3201/eid1407.080017. PMC 2600324. PMID 18598643.
- James R, Barratt J, Marriott D, Harkness J, Stark D (October 2010). "Seroprevalence of Entamoeba histolytica infection among men who have sex with men in Sydney, Australia". The American Journal of Tropical Medicine and Hygiene. 83 (4): 914–6. doi:10.4269/ajtmh.2010.10-0231. PMC 2946768. PMID 20889891.
- Hung CC, Deng HY, Hsiao WH, Hsieh SM, Hsiao CF, Chen MY, et al. (February 2005). "Invasive amebiasis as an emerging parasitic disease in patients with human immunodeficiency virus type 1 infection in Taiwan". Archives of Internal Medicine. 165 (4): 409–15. doi:10.1001/archinte.165.4.409. PMID 15738369.
- Loftus B, Anderson I, Davies R, Alsmark UC, Samuelson J, Amedeo P, et al. (February 2005). "The genome of the protist parasite Entamoeba histolytica" (PDF). Nature. 433 (7028): 865–8. Bibcode:2005Natur.433..865L. doi:10.1038/nature03291. PMID 15729342.
- Lorenzi HA, Puiu D, Miller JR, Brinkac LM, Amedeo P, Hall N, Caler EV (June 2010). "New assembly, reannotation and analysis of the Entamoeba histolytica genome reveal new genomic features and protein content information". PLoS Neglected Tropical Diseases. 4 (6): e716. doi:10.1371/journal.pntd.0000716. PMC 2886108. PMID 20559563.
- Caler, E & Lorenzi, H (2010). "Entamoeba histolytica: Genome Status and Web Resources". Anaerobic Parasitic Protozoa: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-61-5.
- Bakre AA, Rawal K, Ramaswamy R, Bhattacharya A, Bhattacharya S (July 2005). "The LINEs and SINEs of Entamoeba histolytica: comparative analysis and genomic distribution". Experimental Parasitology. 110 (3): 207–13. doi:10.1016/j.exppara.2005.02.009. PMID 15955314.
- Yadav VP, Mandal PK, Rao DN, Bhattacharya S (December 2009). "Characterization of the restriction enzyme-like endonuclease encoded by the Entamoeba histolytica non-long terminal repeat retrotransposon EhLINE1". The FEBS Journal. 276 (23): 7070–82. doi:10.1111/j.1742-4658.2009.07419.x. PMID 19878305.
- "Pathogenic Properties of Some Common Pathogens" (PDF). MtSac.edu. Professor Cindy Anderson. Retrieved 24 October 2017.
- Brown M, Reed S, Levy JA, Busch M, McKerrow JH (January 1991). "Detection of HIV-1 in Entamoeba histolytica without evidence of transmission to human cells". AIDS. 5 (1): 93–6. doi:10.1097/00002030-199101000-00014. PMID 2059366.
- Diamond LS, Mattern CF, Bartgis IL (February 1972). "Viruses of Entamoeba histolytica. I. Identification of transmissible virus-like agents". Journal of Virology. 9 (2): 326–41. PMC 356300. PMID 4335522.
- Ghosh S, Padalia J, Moonah S (21 January 2019). ": Cell Death, Inflammation, Invasion, and the Gut Microbiome". Current Clinical Microbiology Reports. 6 (1): 51–57. doi:10.1007/s40588-019-0113-6. PMC 6449278. PMID 31008019.
- "Entamoeba histolytica". cdc.gov. Centers for Disease Control. Retrieved 24 October 2017.
- "Entamoeba histolytica". Centers for Disease Control & Prevention. CDC.gov. Retrieved 24 October 2017.
- Kucik CJ, Martin GL, Sortor BV (March 2004). "Common intestinal parasites". American Family Physician. 69 (5): 1161–8. PMID 15023017.
- Blessmann J, Tannich E (October 2002). "Treatment of asymptomatic intestinal Entamoeba histolytica infection". The New England Journal of Medicine. 347 (17): 1384. doi:10.1056/NEJM200210243471722. PMID 12397207.
- Stanley SL (March 2003). "Amoebiasis". Lancet. 361 (9362): 1025–34. doi:10.1016/S0140-6736(03)12830-9. PMID 12660071.
- "Diloxanide (Systemic)". Retrieved 17 November 2011.
- Kelso AA, Say AF, Sharma D, Ledford LL, Turchick A, Saski CA, et al. (2015). "Entamoeba histolytica Dmc1 Catalyzes Homologous DNA Pairing and Strand Exchange That Is Stimulated by Calcium and Hop2-Mnd1". PLOS ONE. 10 (9): e0139399. Bibcode:2015PLoSO..1039399K. doi:10.1371/journal.pone.0139399. PMC 4589404. PMID 26422142.
- López-Casamichana M, Orozco E, Marchat LA, López-Camarillo C (April 2008). "Transcriptional profile of the homologous recombination machinery and characterization of the EhRAD51 recombinase in response to DNA damage in Entamoeba histolytica". BMC Molecular Biology. 9: 35. doi:10.1186/1471-2199-9-35. PMC 2324109. PMID 18402694.
- Singh N, Bhattacharya A, Bhattacharya S (2013). "Homologous recombination occurs in Entamoeba and is enhanced during growth stress and stage conversion". PLOS ONE. 8 (9): e74465. Bibcode:2013PLoSO...874465S. doi:10.1371/journal.pone.0074465. PMC 3787063. PMID 24098652.
External links
| Wikimedia Commons has media related to Entamoeba histolytica. |
- Entamoeba histolytica image library
- Entamoeba histolytica - Centers for Disease Control and Prevention
- CDC DPDx Parasitology Diagnostic Web Site
- LSHTM 'Entamoeba Homepage
- 'Entamoeba' Genome Resource - AmoebaDB
- Entamoeba histolytica article from the US Food and Drug Administration's Bad Bug Book