Endothelium
Endothelium refers to cells that line the interior surface of blood vessels and lymphatic vessels,[1] forming an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. It is a thin layer of simple, or single-layered, squamous cells called endothelial cells. Endothelial cells in direct contact with blood are called vascular endothelial cells, whereas those in direct contact with lymph are known as lymphatic endothelial cells.
| Endothelium | |
|---|---|
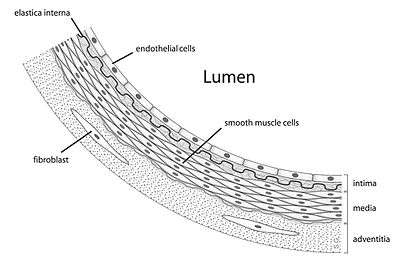 Diagram showing the location of endothelial cells | |
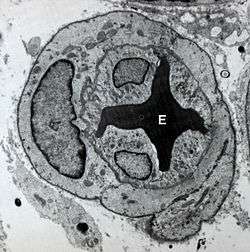 Transmission electron micrograph of a microvessel showing endothelial cells, which encircle an erythrocyte (E), forming the innermost layer of the vessel, the tunica intima. | |
| Details | |
| System | Circulatory system |
| Location | Lining of the inner surface of blood vessels and lymphatic vessels |
| Identifiers | |
| MeSH | D004727 |
| TH | H2.00.02.0.02003 |
| FMA | 63916 |
| Anatomical terms of microanatomy | |
Vascular endothelial cells line the entire circulatory system, from the heart to the smallest capillaries. These cells have unique functions in vascular biology. These functions include fluid filtration, such as in the glomerulus of the kidney, blood vessel tone, hemostasis, neutrophil recruitment, and hormone trafficking. Endothelium of the interior surfaces of the heart chambers is called endocardium.
Structure
Endothelium is of mesodermal origin. Both blood and lymphatic capillaries are composed of a single layer of endothelial cells called a monolayer. In straight sections of a blood vessel, vascular endothelial cells typically align and elongate in the direction of fluid flow.[2][3]
Terminology
The foundational model of anatomy makes a distinction between endothelial cells and epithelial cells on the basis of which tissues they develop from, and states that the presence of vimentin rather than keratin filaments separates these from epithelial cells.[4] Many considered the endothelium a specialized epithelial tissue.[5]
Function
Endothelial cells are involved in many aspects of vascular biology, including:
- Barrier function - the endothelium acts as a semi-selective barrier between the vessel lumen and surrounding tissue, controlling the passage of materials and the transit of white blood cells into and out of the bloodstream. Excessive or prolonged increases in permeability of the endothelial monolayer, as in cases of chronic inflammation, may lead to tissue edema/swelling. Altered barrier function is also implicated in cancer extravasation.[6]
- Blood clotting (thrombosis & fibrinolysis). The endothelium normally provides a non-thrombogenic surface because it contains, for example, heparan sulfate which acts as a cofactor for activating antithrombin, a protease that inactivates several factors in the coagulation cascade.
- Inflammation[7]. Endothelial cells actively signal to immune cells[8] during inflammation
- Formation of new blood vessels (angiogenesis)
- Vasoconstriction and vasodilation, and hence the control of blood pressure
- Repair of damaged or diseased organs via an injection of blood vessel cells[9]
- Angiopoietin-2 works with VEGF to facilitate cell proliferation and migration of endothelial cells
Angiogenesis is a crucial process for embryonic and fetal development and organ growth[10]. The process is triggered by tissue hypoxia or insufficient oxygen tension leading to the new development of blood vessels lined with endothelial cells. Angiogenesis is a tightly regulated event that is balanced by pro- and antiangiogenic signals including integrins, chemokines, angiopoietins, oxygen sensing agents, junctional molecules and endogenous inhibitors.[11]
The general outline of the process is
- activating signals binding to surface receptors of vascular endothelial cells.
- activated endothelial cells release proteases leading to the degradation of the basement membrane
- endothelial cells are freed to migrate from the existing blood vessels and begin to proliferate to form extensions towards the source of the angiogenic stimulus.
Clinical significance
Endothelial dysfunction, or the loss of proper endothelial function, is a hallmark for vascular diseases, and is often regarded as a key early event in the development of atherosclerosis. Impaired endothelial function, causing hypertension and thrombosis, is often seen in patients with coronary artery disease, diabetes mellitus, hypertension, hypercholesterolemia, as well as in smokers. Endothelial dysfunction has also been shown to be predictive of future adverse cardiovascular events, and is also present in inflammatory disease such as rheumatoid arthritis and systemic lupus erythematosus.
Endothelial dysfunction is a result of changes in endothelial physiology[12][13]. In response to lipid accumulation and proinflammatory stimuli, endothelial cells become activated, which is characterized by the expression of adhesion molecules such as E-selectin, VCAM-1 and ICAM-1[14]. Additionally, transcription factors including AP-1 and NF-κB become activated, leading to up-regulated expression of proinflammatory cytokines, such as IL-1, TNFα and IFNγ[15][16]. The proatherogenic profile expressed by the endothelial cells promotes accumulation of lipids and lipoproteins in the intima, and subsequent recruitment of leukocytes and platelets, as well as proliferation of smooth muscle cells, leading to fatty streak formation. The lesions formed in the intima, and persistent inflammation lead to desquamation of endothelium, which disrupts the endothelial barrier, leading to injury and consequent dysfunction[17]. In contrast, inflammatory stimuli also activate NF-κB induced expression of the deubiquitinase A20 (TNFAIP3), which has been shown to intrinsically repair the endothelial barrier [18].
One of the main mechanisms of endothelial dysfunction is the diminishing of nitric oxide, often due to high levels of asymmetric dimethylarginine, which interfere with the normal L-arginine-stimulated nitric oxide synthesis and so leads to hypertension. The most prevailing mechanism of endothelial dysfunction is an increase in reactive oxygen species, which can impair nitric oxide production and activity via several mechanisms.[19] The signalling protein ERK5 is essential for maintaining normal endothelial cell function.[20] A further consequence of damage to the endothelium is the release of pathological quantities of von Willebrand factor, which promote platelet aggregation and adhesion to the subendothelium, and thus the formation of potentially fatal thrombi.
In cancer therapy the development and delivery of anti-angiogenic drugs is a very promising path and restoring vascular homeostasis holds great potential for the treatment of ischemic tissue diseases [21]
In August 2019, a mouse model study led by Joshua Scallan, PhD, assistant professor of molecular pharmacology and physiology at USF Health Morsani College of Medicine, identified never before seen cellular processes controlling development of the small valves inside lymphatic vessels, which prevent lymph fluid from flowing the wrong way back into tissues. According to the study, the one-way valves work with muscles to help propel lymph fluid through the body and regulate flow. These groundbreaking findings suggest that targeting the signaling pathways involved in creating and maintaining lymphatic valves may lead to a viable therapy option for patients diagnosed with lymphedema. [22]
History
In 1958 Todd demonstrated that endothelium in human blood vessels have fibrinolytic activity.[23][24]
See also
- Apelin
- Caveolae
- Cellular dewetting
- Endothelial activation
- Endothelial microparticle
- Endothelial progenitor cell
- Endothelium-derived relaxing factor (EDRF)
- Robert F. Furchgott (1998 Nobel prize for discovery of EDRF)
- Platelet activation
- Susac's syndrome
- Tunica intima
- VE-cadherin
- Weibel–Palade body
- Angiocrine growth factors
References
- "Endothelium" at Dorland's Medical Dictionary
- Eskin SG, Ives CL, McIntire LV, Navarro LT (July 1984). "Response of cultured endothelial cells to steady flow". Microvascular Research. 28 (1): 87–94. doi:10.1016/0026-2862(84)90031-1. PMID 6748961.
- Langille BL, Adamson SL (April 1981). "Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice". Circulation Research. 48 (4): 481–8. doi:10.1161/01.RES.48.4.481. PMID 7460219.
- "Endothelial cell". BioPortal. Stanford University. Archived from the original on 2013-10-02. Retrieved 2013-09-28.
- Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V (April 2012). "Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease". Circulation. 125 (14): 1795–808. doi:10.1161/circulationaha.111.040352. PMC 3333843. PMID 22492947.
- Escribano J, Chen MB, Moeendarbary E, Cao X, Shenoy V, Garcia-Aznar JM, et al. (May 2019). "Balance of mechanical forces drives endothelial gap formation and may facilitate cancer and immune-cell extravasation". PLoS Computational Biology. 15 (5): e1006395. doi:10.1371/journal.pcbi.1006395. PMC 6497229. PMID 31048903.
- Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, et al. (June 2016). "Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation". Arteriosclerosis, Thrombosis, and Vascular Biology. 36 (6): 1090–100. doi:10.1161/ATVBAHA.115.306964. PMC 4882253. PMID 27127201.
- Vestweber D (November 2015). "How leukocytes cross the vascular endothelium". Nature Reviews. Immunology. 15 (11): 692–704. doi:10.1038/nri3908. PMID 26471775.
- "Blood vessel cells can repair, regenerate organs, scientists say". Retrieved 2018-11-13.
- Bouïs D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA (February 2006). "A review on pro- and anti-angiogenic factors as targets of clinical intervention". Pharmacological Research. 53 (2): 89–103. doi:10.1016/j.phrs.2005.10.006. PMID 16321545.
- Bouïs D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA (February 2006). "A review on pro- and anti-angiogenic factors as targets of clinical intervention". Pharmacological Research. 53 (2): 89–103. doi:10.1016/j.phrs.2005.10.006. PMID 16321545.
- Iantorno M, Campia U, Di Daniele N, Nistico S, Forleo GB, Cardillo C, Tesauro M (April 2014). "Obesity, inflammation and endothelial dysfunction". Journal of Biological Regulators and Homeostatic Agents. 28 (2): 169–76. PMID 25001649.
- Reriani MK, Lerman LO, Lerman A (June 2010). "Endothelial function as a functional expression of cardiovascular risk factors". Biomarkers in Medicine. 4 (3): 351–60. doi:10.2217/bmm.10.61. PMC 2911781. PMID 20550469.
- Lopez-Garcia E, Hu FB (August 2004). "Nutrition and the endothelium". Current Diabetes Reports. 4 (4): 253–9. doi:10.1007/s11892-004-0076-7. PMID 15265466.
- Blake GJ, Ridker PM (October 2002). "Inflammatory bio-markers and cardiovascular risk prediction". Journal of Internal Medicine. 252 (4): 283–94. doi:10.1046/j.1365-2796.2002.01019.x. PMID 12366601.
- Mizuno Y, Jacob RF, Mason RP (2011). "Inflammation and the development of atherosclerosis". Journal of Atherosclerosis and Thrombosis. 18 (5): 351–8. doi:10.5551/jat.7591. PMID 21427505.
- Mäyränpää MI, Heikkilä HM, Lindstedt KA, Walls AF, Kovanen PT (November 2006). "Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion". Coronary Artery Disease. 17 (7): 611–21. doi:10.1097/01.mca.0000224420.67304.4d. PMID 17047445.
- Soni D, Wang DM, Regmi SC, Mittal M, Vogel SM, Schlüter D, Tiruppathi C (May 2018). "Deubiquitinase function of A20 maintains and repairs endothelial barrier after lung vascular injury". Cell Death Discovery. 4 (60): 60. doi:10.1038/s41420-018-0056-3. PMC 5955943. PMID 29796309.
- Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ (January 2005). "Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension". Journal of Hypertension. 23 (1): 7–17. doi:10.1097/00004872-200501000-00004. PMID 15643116.
- Roberts OL, Holmes K, Müller J, Cross DA, Cross MJ (December 2009). "ERK5 and the regulation of endothelial cell function". Biochemical Society Transactions. 37 (Pt 6): 1254–9. doi:10.1042/BST0371254. PMID 19909257.
- Van Hove AH, Benoit DS (2015). "Depot-Based Delivery Systems for Pro-Angiogenic Peptides: A Review". Frontiers in Bioengineering and Biotechnology. 3: 102. doi:10.3389/fbioe.2015.00102. PMC 4504170. PMID 26236708.
- Ying Y, Boksik C, Motawe ZY, Srinivasan R, Scallan J (2019). "VE-Cadherin Is Required for Lymphatic Valve Formation and Maintenance". Cell Reports. 28 (9): 2397–2412.E4. doi:10.1016/j.celrep.2019.07.072. PMC 6743082. PMID 31461654.
- Todd, AS (15 February 1958). "Fibrinolysis autographs". Nature. 181 (4607): 495–496. doi:10.1038/181495b0. eISSN 1476-4687. ISSN 0028-0836. PMID 13517190.
- Todd, AS (1 September 1964). "Localization of fibrinolytic activity in tissues" (PDF). British Medical Bulletin. 20 (3): 210–212. doi:10.1093/oxfordjournals.bmb.a070333. eISSN 1471-8391. ISSN 0007-1420. PMID 14209761.
External links
- Anatomy photo: Circulatory/vessels/capillaries1/capillaries3 - Comparative Organology at University of California, Davis, "Capillaries, non-fenestrated (EM, Low)"
- Histology image: 21402ooa – Histology Learning System at Boston University
- Endothelium Journal of Endothelial Cell Research, Informa Healthcare
- Endothelium and inflammation
- Platelet Activation, University of Washington