Enasidenib
Enasidenib (INN; trade name Idhifa) is a drug used to treat relapsed or refractory acute myeloid leukemia in people with specific mutations of the isocitrate dehydrogenase 2 (IDH2) gene, determined by an FDA-approved IDH2 companion diagnostic test.[1] It is an inhibitor of IDH2. It was developed by Agios Pharmaceuticals and is licensed to Celgene for further development.
 | |
| Clinical data | |
|---|---|
| Trade names | Idhifa |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
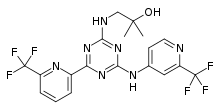
| Formula | C19H17F6N7O |
| Molar mass | 473.383 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Medical use
Enasidenib is used to treat relapsed or refractory acute myeloid leukemia in people with specific mutations of the IDH2 gene, determined by an FDA-approved IDH2 companion diagnostic test.[1]
Adverse effects
The main serious adverse effect of enasidenib is differentiation syndrome.[2]
Pharmacology
Isocitrate dehydrogenase is a critical enzyme in the citric acid cycle. Mutated forms of IDH produce high levels of the (R)-enantiomer of 2-hydroxyglutarate (R-2-HG) and can contribute to the growth of tumors. IDH1 catalyzes this reaction in the cytoplasm, while IDH2 catalyzes this reaction in mitochondria. Mutations of IDH2 are more common than IDH1 mutations, 8 to 19% compared to 7 to 14% respectively,[1] in those affected with AML. Enasidenib disrupts this cycle by decreasing total (R)-2-HG levels in the mitochondria.
History
The FDA granted fast track designation and orphan drug status for acute myeloid leukemia in 2014.[2]
Enasidenib was approved by the FDA in August 2017 for relapsed or refractory acute myeloid leukemia (AML) in people with specific mutations of the IDH2 gene, determined by an FDA-approved IDH2 companion diagnostic test.[1][3][4]
References
- Kim, ES (6 September 2017). "Enasidenib: First Global Approval". Drugs. 77 (15): 1705. doi:10.1007/s40265-017-0813-2. PMID 28879540.
- Brunton, Laurence L.; Hilal-Dandan, Randa; Knollmann, Björn C. (eds.). Goodman & Gilman's the pharmacological basis of therapeutics (13th ed.). New York. ISBN 9781259584732. OCLC 993810322.
- "FDA Approves New Treatment for Leukemia". GEN. August 2, 2017.
- "Press release: FDA granted regular approval to enasidenib for the treatment of relapsed or refractory AML". FDA. August 1, 2017.