Elobixibat
Elobixibat is an inhibitor of the ileal bile acid transporter (IBAT),[1] undergoing development in clinical trials for the treatment of chronic constipation and irritable bowel syndrome with constipation (IBS-C).
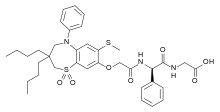 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C36H45N3O7S2 |
| Molar mass | 695.89 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mechanism of action
IBAT is the bile acid:sodium symporter responsible for the reuptake of bile acids in the ileum which is the initial step in the enterohepatic circulation. By inhibiting the uptake of bile acids, elobixibat increases the bile acid concentration in the gut, and this accelerates intestinal passage and softens the stool. Following several phase II studies, it is now undergoing phase III trials.[2]
Drug development
The drug was developed by Albireo AB, who licensed it to Ferring Pharmaceuticals for further development and marketing.[3] Albireo has partnered with Ajinomoto Pharmaceuticals, giving the Japan-based company the rights to further develop the drug and market it throughout Asia.[4]
References
- "INN for A3309 is ELOBIXIBAT". AlbireoPharma. Archived from the original on 18 January 2012. Retrieved 5 December 2012.
- Acosta A, Camilleri M (2014). "Elobixibat and its potential role in chronic idiopathic constipation". Therap Adv Gastroenterol. 7 (4): 167–75. doi:10.1177/1756283X14528269. PMC 4107709. PMID 25057297.
- Grogan, Kevin. "Ferring acquires rights to Albireo's bowel drug". PharmaTimes. Retrieved 23 March 2017.
- "Ajinomoto Pharmaceuticals and Albireo Announce Japan and Asia License Agreement for Elobixibat". Albireo. Retrieved 5 December 2012.