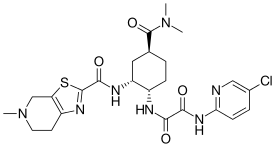
Edoxaban
Edoxaban (trade names Savaysa, Lixiana) is an oral anticoagulant drug and a direct factor Xa inhibitor. Compared with warfarin it has fewer drug interactions.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Savaysa, Lixiana, others |
| Other names | DU-176b |
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 62%; Tmax 1–2 hours[1] |
| Protein binding | 55%[1] |
| Metabolism | minimal CES1, CYP3A4/5, hydrolysis, glucuronidation[1] |
| Elimination half-life | 10–14 hours[1] |
| Excretion | 62% feces, 35% urine (97% of 60 mg)[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H30ClN7O4S |
| Molar mass | 548.06 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It was developed by Daiichi Sankyo and approved in July 2011 in Japan for prevention of venous thromboembolisms following lower-limb orthopedic surgery.[2] It was also approved in the US by the FDA in January 2015 for the prevention of stroke and non–central-nervous-system systemic embolism.[3] It was approved for use in the EU in June 2015.[4]
Medical uses
In the US, edoxaban is approved for treating deep vein thrombosis and pulmonary embolism following 5 to 10 days of initial therapy with a parenteral anticoagulant. It is also approved for reducing the risk of blood clots in patients with nonvalvular atrial fibrillation.[5]
In the EU, edoxaban is approved for preventing blood clots in patients with nonvalvular atrial fibrillation who also have at least one risk factor, such as having had a previous stroke, high blood pressure, diabetes mellitus, heart failure or being 75 years old or over. It is also used to treat deep vein thrombosis and pulmonary embolism and to prevent either of these from reoccurring.[4]
Doses are often taken regularly and with or without food. Typical doses for clot prevention are 60 milligrams (mg) once a day for adults, but 30 mg doses may be used in people who weight less than 60 kilograms or have moderate kidney failure. 15 mg daily doses may be used when transitioning between edoxaban and some other anticoagulant.[5][6]
Contraindications and notes
Edoxaban is often contraindicated in people (incomplete list):
- with active pathological bleeding[6]
- who are pregnant or breastfeeding[6]
- who have conditions that increase bleeding risks. Examples: liver disease associated with coagulopathy and relevant bleeding risk, current or recent gastrointestinal ulceration, malignant neoplasms at high risk of bleeding, recent brain injury or spinal injury, recent brain, spinal or ophthalmic surgery, known or suspected esophageal varices, arteriovenous malformations, aneurysms or major intraspinal or intracerebral vascular abnormalities[6]
- who have uncontrolled and severe high blood pressure[6]
- who use any other anticoagulants[6]
Edoxaban (incomplete list):
- is enhanced by the following strong P-glycoprotein (P-gp) inhibitors: ciclosporin, dronedarone, erythromycin or ketoconazole. Concomittant use of these and edoxaban may require 30 mg doses of edoxaban (instead of 60 mg). The efficacy of edoxaban may decrease when used ith with strong P-gp inducers like phenytoin, carbamazepine, phenobarbital or St. John's Wort, and should be used with caution.[6]
- interacts with antiplatelets, NSAIDs and SSRIs and SNRIs.[6]
- works less well than warfarin in nonvalvular atrial fibrillation with a creatinine clearance (CrCl) greater than 95 ml/min.[5]
Adverse effects
May affect up to 1 in 10 people:[6]
- stomach ache
- abnormal liver blood tests
- anemia
- bleeding from the skin, nose, vagina, bowel, mouth, throat or stomach
- rash
- bloody urine
- dizziness
- feeling sick
- headache
- itching
May affect up to 1 in 100 people:[6]
- bleeding in the eyes, brain, after a surgical operation or other types of bleeding
- blood in the spit when coughing
- reduced number of platelets in blood
- allergic reaction
- hives
May affect up to 1 in 1000 people: bleeding in the muscles, joints, abdomen, heart or inside the skull.[6]
Overdose
Edoxaban overdose can cause serious bleeding. No FDA or EMA approved antidotes for edoxaban overdose exist. Hemodialysis does not significantly contribute to edoxaban clearance.[5][6] Andexanet alfa has been studied as an antidote for edoxaban overdose, but has only been approved for reversing rivaroxaban and apixaban effects by the FDA and the EMA as of 2019.[7][8]
Mechanism of action
Edoxaban is a direct, selective, reversible and competitive inhibitor of human factor Xa, with an inhibitory constant (Ki) value of 0.561 nM. In coagulation, uninhibited factor Xa forms a prothrombinase complex with factor Va on platelet surfaces. Prothrombinases turn prothrombins to thrombins. Thrombins turn blood-soluble fibrinogens to insoluble fibrins, which are the main components of blood clots.[1]
Pharmacokinetics
In human, 15–150 mg oral doses of edoxaban reach their maximum concentrations in blood 1–2 hours after ingestion. With 60 mg doses of isotope labeled edoxaban, 97% of the total radiation was detected after oral administration, with 62% from feces and 35% from urine. 49% of the total radiation from the feces and 24% from the urine were from edoxaban, the rest from its metabolites.[1]
Metabolism occurs mostly via CES1, CYP3A4, CYP3A5 and enzymatic hydrolysis. CES1 oxidizes the tertiary amide carbonyl carbons of edoxabans to carboxylic acid groups. CYP3A4 and CYP3A5 oxidize edoxabans via hydroxylation or demethylation. In hydrolysis, 2-amino-5-chloropyridine moiety of edoxaban is removed. Glucuronidation occurs to a lesser extend via glucuronosyltransferases.[1]
References
- Parasrampuria DA, Truitt KE (2016). "Pharmacokinetics and pharmacodynamics of edoxaban, a non-vitamin K antagonist oral anticoagulant that inhibits clotting factor Xa". Clinical Pharmacokinetics. 55: 641–655. doi:10.1007/s40262-015-0342-7. PMC 4875962. PMID 26620048.
- "First market approval in Japan for LIXIANA (Edoxaban)". Press Release. Daiichi Sankyo Europe GmbH. 2011-04-22. Archived from the original on 2013-11-06.
- O'Riordan, Michael (9 January 2015). "FDA Approves Edoxaban for Stroke Prevention in AF and DVT/PE Prevention". Medscape. Retrieved 10 January 2015.
- "Lixiana". European Medicines Agency. 2018-09-17. Retrieved 2019-11-06.
- "SAVAYSA (edoxaban) tablets Label" (PDF). Archived (PDF) from the original on 2018-07-27. Retrieved 2019-11-06.
- "Lixiana, INN-edoxaban" (PDF). Archived (PDF) from the original on 2019-11-06. Retrieved 2019-11-06.
- Ovanesov, Mikhail (2017-08-03). "Summary basis for regulatory action - ANDEXXA". Retrieved 2019-11-06.
- "Ondexxya". European Medicines Agency. 2019-02-27. Retrieved 2019-11-06.