NMDA receptor
The N-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel protein found in nerve cells. The NMDA receptor is one of three types of ionotropic glutamate receptors. The other receptors are the AMPA and kainate receptors. It is activated when glutamate and glycine (or D-serine) bind to it, and when activated it allows positively charged ions to flow through the cell membrane.[2] The NMDA receptor is very important for controlling synaptic plasticity and memory function.[3]
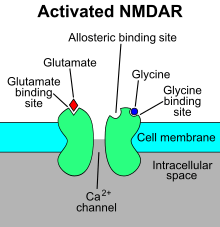
The NMDAR is a specific type of ionotropic glutamate receptor.[4] The NMDA receptor is so named because the agonist molecule N-methyl-D-aspartate (NMDA) binds selectively to it, and not to other glutamate receptors. Activation of NMDA receptors results in the opening of an ion channel that is nonselective to cations, with a combined reversal potential near 0 mV. While the opening and closing of the ion channel is primarily gated by ligand binding, the current flow through the ion channel is voltage dependent. Extracellular magnesium (Mg2+) and zinc (Zn2+) ions can bind to specific sites on the receptor, blocking the passage of other cations through the open ion channel. Depolarization of the cell dislodges and repels the Mg2+ and Zn2+ ions from the pore, thus allowing a voltage-dependent flow of sodium (Na+) and small amounts of calcium (Ca2+) ions into the cell and potassium (K+) out of the cell.[5][6][7][8]
Ca2+ flux through NMDARs is thought to be critical in synaptic plasticity, a cellular mechanism for learning and memory. The opening and closing (gating) of the NMDA receptor is complex. While it is primarily a ligand-gated channel, it does display weaker voltage-dependence modulation of the ligand-dependent gating. The ligand gating requires co-activation by two ligands: glutamate and either D-serine or glycine.[9] The voltage-dependence of current through the channel is mainly due to binding of Mg2+ or Zn2+ ions to the protein as described above.
The activity of the NMDA receptor is affected by many psychoactive drugs such as phencyclidine (PCP), alcohol (ethanol) and dextromethorphan (DXM). The anaesthetic and analgesic effects of the drugs ketamine and nitrous oxide are partially because of their effects on NMDA receptor activity. Since 1989 memantine has been recognized to be an uncompetitive antagonist of the N-methyl-D-aspartate receptor (NMDA receptor), entering the channel of the receptor after it has been activated and thereby blocking the flow of ions.[10][11][12]
The NMDA receptor channels play an important role in synaptic plasticity and synapse formation underlying memory, learning and formation of neural networks during development in the central nervous system (CNS). Overactivation of the receptor, causing excessive influx of Ca2+ can lead to excitotoxicity which is implied to be involved in some neurodegenerative disorders. Blocking of NMDA receptors could therefore, in theory, be useful in treating such diseases.[12][13][14][15]
However, hypofunction of NMDA receptors (due to glutathione deficiency or other causes) may be involved in impairment of synaptic plasticity[16] and could have other negative repercussions. The main problem with the utilization of NMDA receptor antagonists for neuroprotection is that the physiological actions of the NMDA receptor are essential for normal neuronal function. To be clinically accepted, successful clinical application of NMDA antagonists would need to block excessive activation without interfering with normal functions.[17]
History
The discovery of NMDA receptors was followed by the synthesis and study of N-Methyl-D-aspartic acid (NMDA) in the 1960s by Jeff Watkins and colleagues. In the early 1980s, NMDA receptors were shown to be involved in several central synaptic pathways.[18][19] Receptor subunit selectivity was discovered in the early 1990s, which led to recognition of a new class of compounds that selectively inhibit the NR2B subunit. These findings led to vigorous campaign in the pharmaceutical industry.[20] From this it was considered that NMDA receptors were associated with a variety of neurological disorders such as epilepsy, Parkinson's, Alzheimer's, Huntington's and other CNS disorders.[5]
A fortuitous finding was made in 1968 when a woman was taking amantadine as flu medicine and experienced remarkable remission of her Parkinson's symptoms. This finding, reported by Scawab et al., was the beginning of medicinal chemistry of adamantane derivatives in the context of diseases affecting the CNS.[21] Before this finding, memantine, another adamantane derivative, had been synthesized by Eli Lilly and Company in 1963. The purpose was to develop a hypoglycemic drug, but it showed no such efficacy. It was not until 1972 that a possible therapeutic importance of memantine for treating neurodegenerative disorders was discovered. From 1989 memantine has been recognized to be an uncompetitive antagonist of the NMDA receptor.[11]
Properties
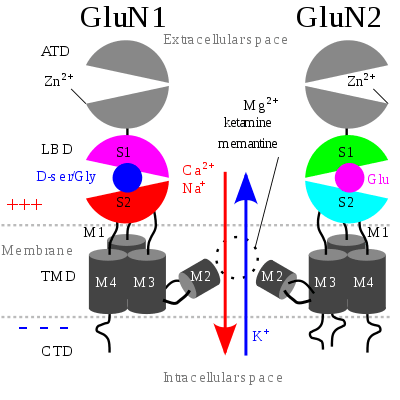
The NMDA receptor is a glutamate and ion channel protein receptor that is activated when glycine and glutamate bind to it.[2] The receptor is a heteromeric complex that interacts with multiple intracellular proteins by three different subunits: GluN1, GluN2 and GluN3. GluN1 has eight different isoforms due to alternative splicing of the gene GRIN1. There are four different GluN2 subunits (A-D) and two different Glun3 subunits (A and B). Six separate genes encode for GluN2 and GluN3.[22][23] All the subunits share a common membrane topology that is dominated by a large extracellular N-terminus, a membrane region comprising three transmembrane segments, a re-entrant pore loop, an extracellular loop between the transmembrane segments that are structurally not well known, and an intracellular C-terminus, which are different in size depending on the subunit and provide multiple sites of interaction with many intracellular proteins.[22][24] Figure 1 shows a basic structure of GluN1/GluN2 subunits that forms the binding site for memantine, Mg2+ and ketamine.
Mg2+ blocks the NMDA receptor channel in a voltage-dependent manner. The channels are also highly permeable to Ca2+. Activation of the receptor depends on glutamate binding, D-serine or glycine binding at its GluN1-linked binding site and AMPA receptor-mediated depolarization of the postsynaptic membrane, which relieves the voltage-dependent channel block by Mg2+. Activation and opening of the receptor channel thus allows the flow of K+, Na+ and Ca2+ ions, and the influx of Ca2+ triggers intracellular signaling pathways.[10][25] Allosteric receptor binding sites for zinc, proteins and the polyamines spermidine and spermine are also modulators for the NMDA receptor channels.[26]
The GluN2B subunit has been involved in modulating activity such as learning, memory, processing and feeding behaviors, as well as being implicated in number of human derangements. The basic structure and functions associated with the NMDA receptor can be attributed to the GluN2B subunit. For example, the glutamate binding site and the control of the Mg2+ block are formed by the GluN2B subunit. The high affinity sites for glycine antagonist are also exclusively displayed by the GluN1/GluN2B receptor.[23]
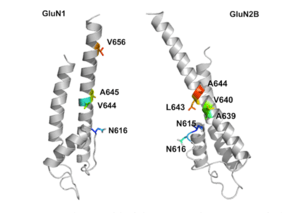
GluN1/GluN2B transmembrane segments are considered to be the part of the receptor that forms the binding pockets for uncompetitive NMDA receptor antagonists, but the transmembrane segments structures are not fully known as stated above. It is claimed that three binding sites within the receptor, A644 on the GluNB subunit and A645 and N616 on the GluN1 subunit, are important for binding of memantine and related compounds as seen in figure 2.[24]
The NMDA receptor forms a heterotetramer between two GluN1 and two GluN2 subunits (the subunits were previously denoted as GluN1 and GluN2), two obligatory GluN1 subunits and two regionally localized GluN2 subunits. A related gene family of GluN3 A and B subunits have an inhibitory effect on receptor activity. Multiple receptor isoforms with distinct brain distributions and functional properties arise by selective splicing of the GluN1 transcripts and differential expression of the GluN2 subunits.
Each receptor subunit has modular design and each structural module, also represents a functional unit:
- The extracellular domain contains two globular structures: a modulatory domain and a ligand-binding domain. GluN1 subunits bind the co-agonist glycine and GluN2 subunits bind the neurotransmitter glutamate.
- The agonist-binding module links to a membrane domain, which consists of three transmembrane segments and a re-entrant loop reminiscent of the selectivity filter of potassium channels.
- The membrane domain contributes residues to the channel pore and is responsible for the receptor's high-unitary conductance, high-calcium permeability, and voltage-dependent magnesium block.
- Each subunit has an extensive cytoplasmic domain, which contain residues that can be directly modified by a series of protein kinases and protein phosphatases, as well as residues that interact with a large number of structural, adaptor, and scaffolding proteins.
The glycine-binding modules of the GluN1 and GluN3 subunits and the glutamate-binding module of the GluN2A subunit have been expressed as soluble proteins, and their three-dimensional structure has been solved at atomic resolution by x-ray crystallography. This has revealed a common fold with amino acid-binding bacterial proteins and with the glutamate-binding module of AMPA-receptors and kainate-receptors.
Mechanism of action
Overactivation of NMDA receptors, relieving the Mg2+ block and causing excessive influx of Ca2+ can lead to excitotoxicity. Excitotoxicity is implied to be involved in some neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease and Huntington's disease.[12][13][14][15] Blocking of NMDA receptors could therefore, in theory, be useful in treating such diseases.[12][13][14] It is, however, important to preserve physiological NMDA receptor activity while trying to block its excessive, excitotoxic activity. This can possibly be achieved by uncompetitive antagonists, blocking the receptors ion channel when excessively open.[14]
Uncompetitive NMDA receptor antagonists, or channel blockers, enter the channel of the NMDA receptor after it has been activated and thereby block the flow of ions.[10][12] MK-801, ketamine, amantadine and memantine are examples of such antagonists,[10] see figure 1. The off-rate of an antagonist from the receptors channel is an important factor as too slow off-rate can interfere with normal function of the receptor and too fast off-rate may give ineffective blockade of an excessively open receptor.[14]
Memantine is an example of an uncompetitive channel blocker of the NMDA receptor, with a relatively rapid off-rate and low affinity. At physiological pH its amine group is positively charged and its receptor antagonism is voltage-dependent.[14] It thereby mimics the physiological function of Mg2+ as channel blocker.[11] Memantine only blocks NMDA receptor associated channels during prolonged activation of the receptor, as it occurs under excitotoxic conditions, by replacing magnesium at the binding site. During normal receptor activity the channels only stay open for several milliseconds and under those circumstances memantine is unable to bind within the channels and therefore doesn't interfere with normal synaptic activity.[17]
Variants
GluN1
There are eight variants of the GluN1 subunit produced by alternative splicing of GRIN1:[27]
- GluN1-1a, GluN1-1b; GluN1-1a is the most abundantly expressed form.
- GluN1-2a, GluN1-2b;
- GluN1-3a, GluN1-3b;
- GluN1-4a, GluN1-4b;
GluN2
.jpg)
While a single GluN2 subunit is found in invertebrate organisms, four distinct isoforms of the GluN2 subunit are expressed in vertebrates and are referred to with the nomenclature GluN2A through GluN2D (encoded by GRIN2A, GRIN2B, GRIN2C, GRIN2D). Strong evidence shows that the genes encoding the GluN2 subunits in vertebrates have undergone at least two rounds of gene duplication.[28] They contain the binding-site for the neurotransmitter glutamate. More importantly, each GluN2 subunit has a different intracellular C-terminal domain that can interact with different sets of signalling molecules.[29] Unlike GluN1 subunits, GluN2 subunits are expressed differentially across various cell types and developmental timepoints and control the electrophysiological properties of the NMDA receptor. GluN2B is mainly present in immature neurons and in extrasynaptic locations, and contains the binding-site for the selective inhibitor ifenprodil.
GluN2B to GluN2A switch
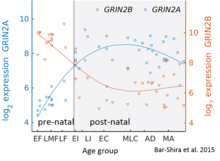
While GluN2B is predominant in the early postnatal brain, the number of GluN2A subunits increases during early development; eventually, GluN2A subunits become more numerous than GluN2B. This is called the GluN2B-GluN2A developmental switch, and is notable because of the different kinetics each GluN2 subunit contributes to receptor function.[31] For instance, greater ratios of the GluN2B subunit leads to NMDA receptors which remain open longer compared to those with more GluN2A.[32] This may in part account for greater memory abilities in the immediate postnatal period compared to late in life, which is the principle behind genetically altered 'doogie mice'. The detailed time course of this switch in the human cerebellum has been estimated using expression microarray and RNA seq and is shown in the figure on the right.
There are three hypothetical models to describe this switch mechanism:
- Increase in synaptic GluN2A along with decrease in GluN2B
- Extrasynaptic displacement of GluN2B away from the synapse with increase in GluN2A
- Increase of GluN2A diluting the number of GluN2B without the decrease of the latter.
The GluN2B and GluN2A subunits also have differential roles in mediating excitotoxic neuronal death.[33] The developmental switch in subunit composition is thought to explain the developmental changes in NMDA neurotoxicity.[34] Homozygous disruption of the gene for GluN2B in mice causes perinatal lethality, whereas disruption of the GluN2A gene produces viable mice, although with impaired hippocampal plasticity.[35] One study suggests that reelin may play a role in the NMDA receptor maturation by increasing the GluN2B subunit mobility.[36]
GluN2B to GluN2C switch
Granule cell precursors (GCPs) of the cerebellum, after undergoing symmetric cell division[37] in the external granule-cell layer (EGL), migrate into the internal granule-cell layer (IGL) where they downregulate GluN2B and activate GluN2C, a process that is independent of neuregulin beta signaling through ErbB2 and ErbB4 receptors.[38]
Role in excitotoxicity
NMDA receptors have been implicated by a number of studies to be strongly involved with excitotoxicity.[39][40][41] Because NMDA receptors play an important role in the health and function of neurons, there has been much discussion on how these receptors can affect both cell survival and cell death.[42] Recent evidence supports the hypothesis that overstimulation of extrasynaptic NMDA receptors has more to do with excitotoxicity than stimulation of their synaptic counterparts.[39][43] In addition, while stimulation of extrasynaptic NMDA receptors appear to contribute to cell death, there is evidence to suggest that stimulation of synaptic NMDA receptors contributes to the health and longevity of the cell. There is ample evidence to support the dual nature of NMDA receptors based on location, and the hypothesis explaining the two differing mechanisms is known as the "localization hypothesis".[39][42]
Differing cascade pathways
In order to support the localization hypothesis, it would be necessary to show differing cellular signaling pathways are activated by NMDA receptors based on its location within the cell membrane.[39] Experiments have been designed to stimulate either synaptic or non-synaptic NMDA receptors exclusively. These types of experiments have shown that different pathways are being activated or regulated depending on the location of the signal origin.[44] Many of these pathways use the same protein signals, but are regulated oppositely by NMDARs depending on its location. For example, synaptic NMDA excitation caused a decrease in the intracellular concentration of p38 mitogen-activated protein kinase (p38MAPK). Extrasynaptic stimulation NMDARs regulated p38MAPK in the opposite fashion, causing an increase in intracellular concentration.[45][46] Experiments of this type have since been repeated with the results indicating these differences stretch across many pathways linked to cell survival and excitotoxicity.[39]
Two specific proteins have been identified as a major pathway responsible for these different cellular responses ERK1/2, and Jacob.[39] ERK1/2 is responsible for phosphorylation of Jacob when excited by synaptic NMDARs. This information is then transported to the nucleus. Phosphorylation of Jacob does not take place with extrasynaptic NMDA stimulation. This allows the transcription factors in the nucleus to respond differently based in the phosphorylation state of Jacob.[47]
Neural plasticity
NMDA receptors are also associated with synaptic plasticity. The idea that both synaptic and extrasynaptic NMDA receptors can affect long-term potentiation (LTP) and long-term depression (LTD) differently has also been explored.[39][48] Experimental data suggest that extrasynaptic NMDA receptors inhibit LTP while producing LTD.[49] Inhibition of LTP can be prevented with the introduction of a NMDA antagonist.[39] A theta burst stimulation that usually induces LTP with synaptic NMDARs, when applied selectively to extrasynaptic NMDARs produces a LTD.[50] Experimentation also indicates that extrasynaptic activity is not required for the formation of LTP. In addition, both synaptic and extrasynaptic are involved in expressing a full LTD.[51]
Role of differing subunits
Another factor that seems to affect NMDAR induced toxicity is the observed variation in subunit makeup. NMDA receptors are heterotetramers with two GluN1 subunits and two variable subunits.[39][52] Two of these variable subunits, GluN2A and GluN2B, have been shown to preferentially lead to cell survival and cell death cascades respectively. Although both subunits are found in synaptic and extrasynaptic NMDARs there is some evidence to suggest that the GluN2B subunit occurs more frequently in extrasynaptic receptors. This observation could help explain the dualistic role that NMDA receptors play in excitotoxicity.[53][54]
Despite the compelling evidence and the relative simplicity of these two theories working in tandem, there is still disagreement about the significance of these claims. Some problems in proving these theories arise with the difficulty of using pharmacological means to determine the subtypes of specific NMDARs.[39][55] In addition, the theory of subunit variation does not explain how this effect might predominate, as it is widely held that the most common tetramer, made from two GluN1 subunits and one of each subunit GluN2A and GluN2B, makes up a high percentage of the NMDARs.[39]
Excitotoxicity in a clinical setting
Excitotoxicity has been thought to play a role in the degenerative properties of neurodegenerative conditions since the late 1950s.[56] NMDA receptors seem to play an important role in many of these degenerative diseases affecting the brain. Most notably, excitotoxic events involving NMDA receptors have been linked to Alzheimer's disease and Huntington's disease, as well as with other medical conditions such as strokes and epilepsy.[39][57] Treating these conditions with one of the many known NMDA receptor antagonists, however, leads to a variety of unwanted side effects, some of which can be severe. These side effects are, in part, observed because the NMDA receptors do not just signal for cell death but also play an important role in its vitality.[42] Treatment for these conditions might be found in blocking NMDA receptors not found at the synapse.[39][58]
Ligands
Agonists

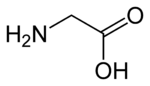
Activation of NMDA receptors requires binding of glutamate or aspartate (aspartate does not stimulate the receptors as strongly).[59] In addition, NMDARs also require the binding of the co-agonist glycine for the efficient opening of the ion channel, which is a part of this receptor.
D-Serine has also been found to co-agonize the NMDA receptor with even greater potency than glycine.[60] It is produced by serine racemase, and is enriched in the same areas as NMDA receptors. Removal of D-serine can block NMDA-mediated excitatory neurotransmission in many areas. Recently, it has been shown that D-serine can be released both by neurons and astrocytes to regulate NMDA receptors.
NMDA receptor (NMDAR)-mediated currents are directly related to membrane depolarization. NMDA agonists therefore exhibit fast Mg2+ unbinding kinetics, increasing channel open probability with depolarization. This property is fundamental to the role of the NMDA receptor in memory and learning, and it has been suggested that this channel is a biochemical substrate of Hebbian learning, where it can act as a coincidence detector for membrane depolarization and synaptic transmission.
Examples
Some known NMDA receptor agonists include:
- Alanine (D-alanine, L-alanine) – endogenous glycine site agonist
- Aspartic acid (aspartate) – endogenous glutamate site agonist
- Glutamic acid (glutamate) – endogenous glutamate site agonist
- Glycine – endogenous glycine site agonist
- Homocysteic acid – endogenous glutamate site agonist
- Ibotenic acid – naturally occurring glutamate site agonist found in Amanita muscaria
- Milacemide – synthetic glycine site agonist; prodrug of glycine
- Quinolinic acid (quinolinate) – endogenous glutamate site agonist
- Sarcosine – endogenous glycine site agonist
- Serine (D-serine, L-serine) – endogenous glycine site agonist
- Spermidine – endogenous polyamine site agonist
- Spermine – endogenous polyamine site agonist
- Tetrazolylglycine – synthetic glutamate site agonist
Positive allosteric modulators include:
- Cerebrosterol – endogenous weak positive allosteric modulator
- Cholesterol – endogenous weak positive allosteric modulator
- Dehydroepiandrosterone (DHEA) – endogenous weak positive allosteric modulator
- Dehydroepiandrosterone sulfate (DHEA-S) – endogenous weak positive allosteric modulator
- Nebostinel (neboglamine) – synthetic positive allosteric modulator of the glycine site
- Pregnenolone sulfate – endogenous weak positive allosteric modulator
Neramexane
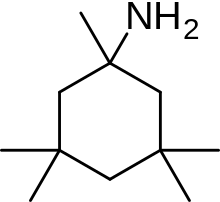
An example of memantine derivative is neramexane which was discovered by studying number of aminoalkyl cyclohexanes, with memantine as the template, as NMDA receptor antagonists. Neramexane, which can be seen in figure 6, binds to the same site as memantine within the NMDA receptor associated channel and with comparable affinity. It does also show very similar bioavailability and blocking kinetics in vivo as memantine. Neramexane went to clinical trials for four indications, including Alzheimer's disease.[21]
Partial agonists
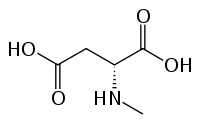
N-Methyl-D-aspartic acid (NMDA), which the NMDA receptor was named after, is a partial agonist of the active or glutamate recognition site.
3,5-Dibromo-L-phenylalanine, a naturally occurring halogenated derivative of L-phenylalanine, is a weak partial NMDA receptor agonist acting on the glycine site.[61] 3,5-Dibromo-L-phenylalanine has been proposed a novel therapeutic drug candidate for treatment of neuropsychiatric disorders and diseases such as schizophrenia,[62] and neurological disorders such as ischemic stroke and epileptic seizures.[63]
Other weak partial agonists of the glycine site of the NMDA receptor such as rapastinel (GLYX-13) and apimostinel (NRX-1074) are now viewed for the development of new drugs with antidepressant and analgesic effects without obvious psychotomimetic activities.[64]
Examples
- Aminocyclopropanecarboxylic acid (ACC) – synthetic glycine site partial agonist
- Cycloserine (D-cycloserine) – naturally occurring glycine site partial agonist found in Streptomyces orchidaceus
- HA-966 – synthetic glycine site weak partial agonist
- Homoquinolinic acid – synthetic glutamate site partial agonist
- N-Methyl-D-aspartic acid (NMDA) – synthetic glutamate site partial agonist
Positive allosteric modulators include:
- Apimostinel (NRX-1074) – synthetic weak partial agonist of an allosteric site of the glycine site
- Rapastinel (GLYX-13) – synthetic weak partial agonist of an allosteric site of the glycine site
Antagonists
Antagonists of the NMDA receptor are used as anesthetics for animals and sometimes humans, and are often used as recreational drugs due to their hallucinogenic properties, in addition to their unique effects at elevated dosages such as dissociation. When certain NMDA receptor antagonists are given to rodents in large doses, they can cause a form of brain damage called Olney's lesions. NMDA receptor antagonists that have been shown to induce Olney's lesions include ketamine, phencyclidine, and dextrorphan (a metabolite of dextromethorphan), as well as some NMDA receptor antagonists used only in research environments. So far, the published research on Olney's lesions is inconclusive in its occurrence upon human or monkey brain tissues with respect to an increase in the presence of NMDA receptor antagonists.[65]
Most NMDAR antagonists are uncompetitive or noncompetitive blockers of the channel pore or are antagonists of the glycine co-regulatory site rather than antagonists of the active/glutamate site.
Examples
Common agents in which NMDA receptor antagonism is the primary or a major mechanism of action:
- 4-Chlorokynurenine (AV-101) – glycine site antagonist; prodrug of 7-chlorokynurenic acid[66][67]
- 7-Chlorokynurenic acid – glycine site antagonist
- Agmatine – endogenous polyamine site antagonist[68][69]
- Argiotoxin-636 – naturally occurring dizocilpine or related site antagonist found in Argiope venom
- AP5 – glutamate site antagonist
- AP7 – glutamate site antagonist
- CGP-37849 – glutamate site antagonist
- Delucemine (NPS-1506) – dizocilpine or related site antagonist; derived from argiotoxin-636[70][71]
- Dextromethorphan (DXM) – dizocilpine site antagonist; prodrug of dextrorphan
- Dextrorphan (DXO) – dizocilpine site antagonist
- Dexanabinol – dizocilpine-related site antagonist[72][73][74]
- Diethyl ether – unknown site antagonist
- Diphenidine – dizocilpine site antagonist
- Dizocilpine (MK-801) – dizocilpine site antagonist
- Eliprodil – ifenprodil site antagonist
- Esketamine – dizocilpine site antagonist
- Hodgkinsine – undefined site antagonist
- Ifenprodil – ifenprodil site antagonist[75]
- Kaitocephalin – naturally occurring glutamate site antagonist found in Eupenicillium shearii
- Ketamine – dizocilpine site antagonist
- Kynurenic acid – endogenous glycine site antagonist
- Lanicemine – low-trapping dizocilpine site antagonist
- LY-235959 – glutamate site antagonist
- Memantine – low-trapping dizocilpine site antagonist
- Methoxetamine – dizocilpine site antagonist
- Midafotel – glutamate site antagonist
- Nitrous oxide (N2O) – undefined site antagonist
- PEAQX – glutamate site antagonist
- Perzinfotel – glutamate site antagonist
- Phencyclidine (PCP) – dizocilpine site antagonist
- Phenylalanine - a naturally occurring amino acid, glycine site antagonist[76][77]
- Psychotridine – undefined site antagonist
- Selfotel – glutamate site antagonist
- Tiletamine – dizocilpine site antagonist
- Traxoprodil – ifenprodil site antagonist
- Xenon – unknown site antagonist
Some common agents in which weak NMDA receptor antagonism is a secondary or additional action include:
- Amantadine – an antiviral and antiparkinsonian drug; low-trapping dizocilpine site antagonist[78]
- Atomoxetine – a norepinephrine reuptake inhibitor used to treat ADHD[79]
- Dextropropoxyphene – an opioid analgesic
- Ethanol (alcohol) – a euphoriant, sedative, and anxiolytic used recreationally; unknown site antagonist
- Guaifenesin – an expectorant
- Huperzine A – a naturally occurring acetylcholinesterase inhibitor and potential antidementia agent
- Ibogaine – a naturally occurring hallucinogen and antiaddictive agent
- Ketobemidone – an opioid analgesic
- Methadone – an opioid analgesic
- Minocycline – an antibiotic[80]
- Tramadol – an atypical opioid analgesic and serotonin releasing agent
Nitromemantine
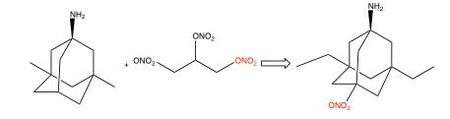
The NMDA receptor is regulated via nitrosylation and aminoadamantane can be used as a target-directed shuttle to bring nitrogen oxide (NO) close to the site within the NMDA receptor where it can nitrosylate and regulate the ion channel conductivity.[21] A NO donor that can be used to decrease NMDA receptor activity is the alkyl nitrate nitroglycerin. Unlike many other NO donors, alkyl nitrates do not have potential NO associated neurotoxic effects. Alkyl nitrates donate NO in the form of a nitro group as seen in figure 7, -NO2-, which is a safe donor that avoids neurotoxicity. The nitro group must be targeted to the NMDA receptor, otherwise other effects of NO such as dilatation of blood vessels and consequent hypotension could result.[81] Nitromemantine is a second-generation derivative of memantine, it reduces excitotoxicity mediated by overactivation of the glutamatergic system by blocking NMDA receptor without sacrificing safety. Provisional studies in animal models show that nitromemantines are more effective than memantine as neuroprotectants, both in vitro and in vivo. Memantine and newer derivatives could become very important weapons in the fight against neuronal damage.[14]
Negative allosteric modulators include:
- 25-Hydroxycholesterol – endogenous weak negative allosteric modulator
- Conantokins – naturally occurring negative allosteric modulators of the polyamine site found in Conus geographus[82]
Modulators
Examples
The NMDA receptor is modulated by a number of endogenous and exogenous compounds:[83]
- Aminoglycosides have been shown to have a similar effect to polyamines, and this may explain their neurotoxic effect.
- CDK5 regulates the amount of NR2B-containing NMDA receptors on the synaptic membrane, thus affecting synaptic plasticity.[84][85]
- Polyamines do not directly activate NMDA receptors, but instead act to potentiate or inhibit glutamate-mediated responses.
- Reelin modulates NMDA function through Src family kinases and DAB1.[86] significantly enhancing LTP in the hippocampus.
- Src kinase enhances NMDA receptor currents.[87]
- Na+, K+ and Ca2+ not only pass through the NMDA receptor channel but also modulate the activity of NMDA receptors.
- Zn2+ and Cu2+ generally block NMDA current activity in a noncompetitive and a voltage-independent manner. However zinc may potentiate or inhibit the current depending on the neural activity.[88]
- Pb2+[89] is a potent NMDAR antagonist. Presynaptic deficits resulting from Pb2+ exposure during synaptogenesis are mediated by disruption of NMDAR-dependent BDNF signaling.
- Proteins of the major histocompatibility complex class I are endogenous negative regulators of NMDAR-mediated currents in the adult hippocampus,[90] and are required for appropriate NMDAR-induced changes in AMPAR trafficking [90] and NMDAR-dependent synaptic plasticity and learning and memory.[91][92]
- The activity of NMDA receptors is also strikingly sensitive to the changes in pH, and partially inhibited by the ambient concentration of H+ under physiological conditions.[93] The level of inhibition by H+ is greatly reduced in receptors containing the NR1a subtype, which contains the positively charged insert Exon 5. The effect of this insert may be mimicked by positively charged polyamines and aminoglycosides, explaining their mode of action.
- NMDA receptor function is also strongly regulated by chemical reduction and oxidation, via the so-called "redox modulatory site."[94] Through this site, reductants dramatically enhance NMDA channel activity, whereas oxidants either reverse the effects of reductants or depress native responses. It is generally believed that NMDA receptors are modulated by endogenous redox agents such as glutathione, lipoic acid, and the essential nutrient pyrroloquinoline quinone.
Development of NMDA receptor antagonists
The main problem with the development of NMDA antagonists for neuroprotection is that physiological NMDA receptor activity is essential for normal neuronal function. Complete blockade of all NMDA receptor activity results in side effects such as hallucinations, agitation and anesthesia. To be clinically relevant, an NMDA receptor antagonist must limit its action to blockade of excessive activation, without limiting normal function of the receptor.[17] Figure 3 shows simplified models of various types of NMDA receptor antagonists, which will be discussed further.
Competitive NMDA receptor antagonists
Competitive NMDA receptor antagonists, which were developed first, are not a good option because they compete and bind to the same site (NR2 subunit) on the receptor as the agonist, glutamate, and therefore block normal function also.[17][95] They will block healthy areas of the brain prior to having an impact on pathological areas, because healthy areas contain lower levels of agonist than pathological areas. These antagonists can be displaced from the receptor by high concentration of glutamate which can exist under excitotoxic circumstances.[12]
Noncompetitive NMDA receptor antagonists
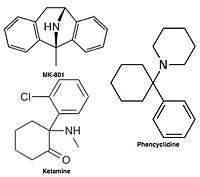
Uncompetitive NMDA receptor antagonists block within the ion channel at the Mg2+ site (pore region) and prevent excessive influx of Ca2+. Noncompetitive antagonism refers to a type of block that an increased concentration of glutamate cannot overcome, and is dependent upon prior activation of the receptor by the agonist, i.e. it only enters the channel when it is opened by agonist.[17][96]
Memantine and related compounds
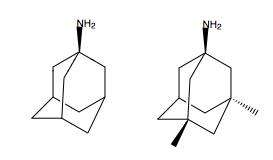
Because of these adverse side effects of high affinity blockers the search for clinically successful NMDA receptor antagonists for neurodegenerative diseases continued and focused on developing low affinity blockers. However the affinity could not be too low and dwell time not too short (as seen with Mg2+) where membrane depolarization relieves the block. The discovery was thereby development of uncompetitive antagonist with longer dwell time than Mg2+ in the channel but shorter than MK-801. That way the drug obtained would only block excessively open NMDA receptor associated channels but not normal neurotransmission.[17][96] Memantine is that drug. It is a derivative of amantadine which was first an anti-influenza agent but was later discovered by coincidence to have efficacy in Parkinson's disease. Chemical structures of memantine and amantadine can be seen in figure 5. The compound was first thought to be dopaminergic or anticholinergic but was later found to be an NMDA receptor antagonist.[11][17]
Memantine is the first drug approved for treatment of severe and more advanced Alzheimer's disease, which for example anticholinergic drugs do not do much good for.[96] It helps recovery of synaptic function and in that way improves impaired memory and learning.[15] In 2015 memantine is also in trials for therapeutic importance in additional neurological disorders.[81]
Many second-generation memantine derivatives have been in development that may show even better neuroprotective effects, where the main thought is to use other safe but effective modulatory sites on the NMDA receptor in addition to its associated ion channel.[81]
Structure activity relationship (SAR)
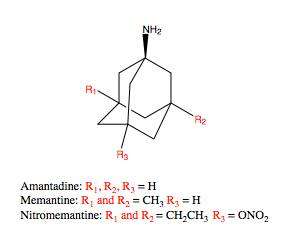
Memantine (1-amino-3,5-dimethyladamantane) is an aminoalkyl cyclohexane derivative and an atypical drug compound with non-planar, three dimensional tricyclic structure. Figure 8 shows SAR for aminoalkyl cyclohexane derivative. Memantine has several important features in its structure for its effectiveness:
- Three-ring structure with a bridgehead amine, -NH2
- The -NH2 group is protonated under physiological pH of the body to carry a positive charge, -NH3+
- Two methyl (CH3) side groups which serve to prolong the dwell time and increase stability as well as affinity for the NMDA receptor channel compared with amantadine (1-adamantanamine).[14][96]
Despite the small structural difference between memantine and amantadine, two adamantane derivatives, the affinity for the binding site of NR1/NR2B subunit is much greater for memantine. In patch-clamp measurements memantine has an IC50 of (2.3+0.3) μM while amantadine has an IC50 of (71.0+11.1) μM.[21] The binding site with the highest affinity is called the dominant binding site. It involves a connection between the amine group of memantine and the NR1-N161 binding pocket of the NR1/NR2B subunit. The methyl side groups play an important role in increasing the affinity to the open NMDA receptor channels and making it a much better neuroprotective drug than amantadine. The binding pockets for the methyl groups are considered to be at the NR1-A645 and NR2B-A644 of the NR1/NR2B.[24] The binding pockets are shown in figure 2. Memantine binds at or near to the Mg2+ site inside the NMDA receptor associated channel. The -NH2 group on memantine, which is protonated under physiological pH of the body, represents the region that binds at or near to the Mg2+ site.[14] Adding two methyl groups to the -N on the memantine structure has shown to decrease affinity, giving an IC50 value of (28.4+1.4) μM.[21]
Second generation derivative of memantine; Nitromemantine
Several derivatives of Nitromemantine, a second-generation derivative of memantine, have been synthesized in order to perform a detailed structure activity relationship (SAR) of these novel drugs. One class, containing a nitro (NO2) group opposite to the bridgehead amine (NH2), showed a promising outcome. Nitromemantine utilizes memantine binding site on the NMDA receptor to target the NOx (X= 1 or 2) group for interaction with the S- nitrosylation/redox site external to the memantine binding site. Lengthening the side chains of memantine compensates for the worse drug affinity in the channel associated with the addition of the –ONO2 group[97]
Therapeutic application
Excitotoxicity is implied to be involved in some neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis.[12][13][14][15] Blocking of NMDA receptors could therefore, in theory, be useful in treating such diseases.[12][13][14] It is, however, important to preserve physiological NMDA receptor activity while trying to block its excessive, excitotoxic activity. This can possibly be achieved by uncompetitive antagonists, blocking the receptors ion channel when excessively open [14]
Memantine is an example of uncompetitive NMDA receptor antagonist that has approved indication for the neurodegenerative disease Alzheimer's disease. In 2015 memantine is still in clinical trials for additional neurological diseases.[24][81]
Receptor modulation
The NMDA receptor is a non-specific cation channel that can allow the passage of Ca2+ and Na+ into the cell and K+ out of the cell. The excitatory postsynaptic potential (EPSP) produced by activation of an NMDA receptor increases the concentration of Ca2+ in the cell. The Ca2+ can in turn function as a second messenger in various signaling pathways. However, the NMDA receptor cation channel is blocked by Mg2+ at resting membrane potential.[98] Magnesium unblock is not instantaneous, to unblock all available channels, the postsynaptic cell must be depolarized for a sufficiently long period of time (in the scale of milliseconds).[99]
Therefore, the NMDA receptor functions as a "molecular coincidence detector". Its ion channel opens only when the following two conditions are met: glutamate is bound to the receptor, and the postsynaptic cell is depolarized (which removes the Mg2+ blocking the channel). This property of the NMDA receptor explains many aspects of long-term potentiation (LTP) and synaptic plasticity.[100]
NMDA receptors are modulated by a number of endogenous and exogenous compounds and play a key role in a wide range of physiological (e.g., memory) and pathological processes (e.g., excitotoxicity).
Clinical significance
NMDAR antagonists like ketamine, esketamine, tiletamine, phencyclidine, nitrous oxide, and xenon are used as general anesthetics. These and similar drugs like dextromethorphan and methoxetamine also produce dissociative, hallucinogenic, and euphoriant effects and are used as recreational drugs.
NMDAR inhibitors, including ketamine, esketamine (JNJ-54135419), rapastinel (GLYX-13), apimostinel (NRX-1074), 4-chlorokynurenine (AV-101), and rislenemdaz (CERC-301, MK-0657), are under development for the treatment of mood disorders, including major depressive disorder and treatment-resistant depression.[66][67][101] In addition, ketamine is already employed for this purpose as an off-label therapy in some clinics.[102][103]
Research suggests that tianeptine produces antidepressant effects through indirect alteration and inhibition of glutamate receptor activity and release of BDNF, in turn affecting neural plasticity.[104][105][106][107][108] Tianeptine also acts on the NMDA and AMPA receptors.[104][108] In animal models, tianeptine inhibits the pathological stress-induced changes in glutamatergic neurotransmission in the amygdala and hippocampus.
Memantine, a low-trapping NMDAR antagonist, is approved in the United States and Europe for the treatment of moderate-to-severe Alzheimer's disease,[109] and has now received a limited recommendation by the UK's National Institute for Health and Care Excellence for patients who fail other treatment options.[110]
Cochlear NMDARs are the target of intense research to find pharmacological solutions to treat tinnitus. NMDARs are associated with a rare autoimmune disease, anti-NMDA receptor encephalitis (a.k.a. NMDAR encephalitis[111]), that usually occurs due to cross-reactivity of antibodies produced by the immune system against ectopic brain tissues, such as those found in teratoma. These are known as anti-glutamate receptor antibodies.
Compared to dopaminergic stimulants like methamphetamine, the NMDAR antagonist phencyclidine can produce a wider range of symptoms that resemble schizophrenia in healthy volunteers, in what has led to the glutamate hypothesis of schizophrenia.[112] Experiments in which rodents are treated with NMDA receptor antagonist are today the most common model when it comes to testing of novel schizophrenia therapies or exploring the exact mechanism of drugs already approved for treatment of schizophrenia.
NMDAR antagonists, for instance eliprodil, gavestinel, licostinel, and selfotel have been extensively investigated for the treatment of excitotoxicity-mediated neurotoxicity in situations like ischemic stroke and traumatic brain injury, but were unsuccessful in clinical trials.
See also
- Calcium/calmodulin-dependent protein kinases
References
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J (March 1997). "Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit". Neuron. 18 (3): 493–503. doi:10.1016/S0896-6273(00)81249-0. PMID 9115742.
Since two molecules of glutamate and glycine each are thought to be required for channel activation (3, 6), this implies that the NMDA receptor should be composed of at least four subunits.
- Furukawa H, Singh SK, Mancusso R, Gouaux E (November 2005). "Subunit arrangement and function in NMDA receptors". Nature. 438 (7065): 185–92. Bibcode:2005Natur.438..185F. doi:10.1038/nature04089. PMID 16281028.
- Li F, Tsien JZ (July 2009). "Memory and the NMDA receptors". The New England Journal of Medicine. 361 (3): 302–3. doi:10.1056/NEJMcibr0902052. PMC 3703758. PMID 19605837.
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S (November 1991). "Molecular cloning and characterization of the rat NMDA receptor". Nature. 354 (6348): 31–7. Bibcode:1991Natur.354...31M. doi:10.1038/354031a0. PMID 1834949.
- Dingledine R, Borges K, Bowie D, Traynelis SF (March 1999). "The glutamate receptor ion channels". Pharmacological Reviews. 51 (1): 7–61. PMID 10049997.
- Liu Y, Zhang J (October 2000). "Recent development in NMDA receptors". Chinese Medical Journal. 113 (10): 948–56. PMID 11775847.
- Cull-Candy S, Brickley S, Farrant M (June 2001). "NMDA receptor subunits: diversity, development and disease". Current Opinion in Neurobiology. 11 (3): 327–35. doi:10.1016/S0959-4388(00)00215-4. PMID 11399431.
- Paoletti P, Neyton J (February 2007). "NMDA receptor subunits: function and pharmacology". Current Opinion in Pharmacology. 7 (1): 39–47. doi:10.1016/j.coph.2006.08.011. PMID 17088105.
- Kleckner NW, Dingledine R (August 1988). "Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes". Science. 241 (4867): 835–7. Bibcode:1988Sci...241..835K. doi:10.1126/science.2841759. PMID 2841759.
- Johnson JW, Kotermanski SE (February 2006). "Mechanism of action of memantine". Current Opinion in Pharmacology. 6 (1): 61–7. doi:10.1016/j.coph.2005.09.007. PMID 16368266.
- Dominguez E, Chin TY, Chen CP, Wu TY (December 2011). "Management of moderate to severe Alzheimer's disease: focus on memantine". Taiwanese Journal of Obstetrics & Gynecology. 50 (4): 415–23. doi:10.1016/j.tjog.2011.10.004. PMID 22212311.
- Chen HS, Lipton SA (June 2006). "The chemical biology of clinically tolerated NMDA receptor antagonists". Journal of Neurochemistry. 97 (6): 1611–26. doi:10.1111/j.1471-4159.2006.03991.x. PMID 16805772.
- Kemp JA, McKernan RM (November 2002). "NMDA receptor pathways as drug targets". Nature Neuroscience. 5 Suppl (11): 1039–42. doi:10.1038/nn936. PMID 12403981.
- Lipton SA (February 2006). "Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond". Nature Reviews. Drug Discovery. 5 (2): 160–70. doi:10.1038/nrd1958. PMID 16424917.
- Koch HJ, Szecsey A, Haen E (1 January 2004). "NMDA-antagonism (memantine): an alternative pharmacological therapeutic principle in Alzheimer's and vascular dementia". Current Pharmaceutical Design. 10 (3): 253–9. doi:10.2174/1381612043386392. PMID 14754385.
- Steullet P, Neijt HC, Cuénod M, Do KQ (February 2006). "Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia". Neuroscience. 137 (3): 807–19. doi:10.1016/j.neuroscience.2005.10.014. PMID 16330153.
- Lipton SA (January 2004). "Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults". NeuroRx. 1 (1): 101–10. doi:10.1602/neurorx.1.1.101. PMC 534915. PMID 15717010.
- Yamakura T, Shimoji K (October 1999). "Subunit- and site-specific pharmacology of the NMDA receptor channel". Progress in Neurobiology. 59 (3): 279–98. doi:10.1016/S0301-0082(99)00007-6. PMID 10465381.
- Watkins JC, Jane DE (January 2006). "The glutamate story". British Journal of Pharmacology. 147 Suppl 1 (S1): S100–8. doi:10.1038/sj.bjp.0706444. PMC 1760733. PMID 16402093.
- Paoletti P, Neyton J (February 2007). "NMDA receptor subunits: function and pharmacology" (PDF). Current Opinion in Pharmacology. 7 (1): 39–47. doi:10.1016/j.coph.2006.08.011. PMID 17088105.
- Wanka L, Iqbal K, Schreiner PR (May 2013). "The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives". Chemical Reviews. 113 (5): 3516–604. doi:10.1021/cr100264t. PMC 3650105. PMID 23432396.
- Loftis JM, Janowsky A (January 2003). "The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications". Pharmacology & Therapeutics. 97 (1): 55–85. doi:10.1016/s0163-7258(02)00302-9. PMID 12493535.
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH (February 2007). "NMDA receptors and schizophrenia". Current Opinion in Pharmacology. 7 (1): 48–55. doi:10.1016/j.coph.2006.08.013. PMID 17097347.
- Limapichat W, Yu WY, Branigan E, Lester HA, Dougherty DA (February 2013). "Key binding interactions for memantine in the NMDA receptor". ACS Chemical Neuroscience. 4 (2): 255–60. doi:10.1021/cn300180a. PMC 3751542. PMID 23421676.
- Maher, T.J. (2013). Anesthetic agents: General and local anesthetics. In: T.L. Lemke & D.A. Williams (editors). Foye's Principles of Medicinal Chemistry. (Chapter 16). Philadelphia: Lippincott Williams & Wilkins
- Danysz W, Parsons CG (September 2003). "The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence". International Journal of Geriatric Psychiatry. 18 (Suppl 1): S23–32. doi:10.1002/gps.938. PMID 12973747.
- Stephenson FA (November 2006). "Structure and trafficking of NMDA and GABAA receptors". Biochemical Society Transactions. 34 (Pt 5): 877–81. doi:10.1042/BST0340877. PMID 17052219.
- Teng H, Cai W, Zhou L, Zhang J, Liu Q, Wang Y, et al. (October 2010). "Evolutionary mode and functional divergence of vertebrate NMDA receptor subunit 2 genes". PLOS ONE. 5 (10): e13342. Bibcode:2010PLoSO...513342T. doi:10.1371/journal.pone.0013342. PMC 2954789. PMID 20976280.
- Ryan TJ, Grant SG (October 2009). "The origin and evolution of synapses". Nature Reviews. Neuroscience. 10 (11): 701–12. doi:10.1038/Nrn2748. PMID 19738623.
- Bar-Shira O, Maor R, Chechik G (December 2015). "Gene Expression Switching of Receptor Subunits in Human Brain Development". PLoS Computational Biology. 11 (12): e1004559. Bibcode:2015PLSCB..11E4559B. doi:10.1371/journal.pcbi.1004559. PMC 4670163. PMID 26636753.
- Liu XB, Murray KD, Jones EG (October 2004). "Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development". The Journal of Neuroscience. 24 (40): 8885–95. doi:10.1523/JNEUROSCI.2476-04.2004. PMC 6729956. PMID 15470155.
- last, first (April 2000). "title". Scientific American.
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, et al. (March 2007). "NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo". The Journal of Neuroscience. 27 (11): 2846–57. doi:10.1523/JNEUROSCI.0116-07.2007. PMC 6672582. PMID 17360906.
- Zhou M, Baudry M (March 2006). "Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors". The Journal of Neuroscience. 26 (11): 2956–63. doi:10.1523/JNEUROSCI.4299-05.2006. PMC 6673978. PMID 16540573.
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, et al. (January 1998). "Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo". Cell. 92 (2): 279–89. doi:10.1016/S0092-8674(00)80921-6. PMID 9458051.
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P (September 2007). "NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin". The Journal of Neuroscience. 27 (38): 10165–75. doi:10.1523/JNEUROSCI.1772-07.2007. PMC 6672660. PMID 17881522.
- Espinosa JS, Luo L (March 2008). "Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells". The Journal of Neuroscience. 28 (10): 2301–12. doi:10.1523/JNEUROSCI.5157-07.2008. PMC 2586640. PMID 18322077.
- Gajendran N, Kapfhammer JP, Lain E, Canepari M, Vogt K, Wisden W, Brenner HR (February 2009). "Neuregulin signaling is dispensable for NMDA- and GABA(A)-receptor expression in the cerebellum in vivo". The Journal of Neuroscience. 29 (8): 2404–13. doi:10.1523/JNEUROSCI.4303-08.2009. PMC 6666233. PMID 19244516.
- Parsons MP, Raymond LA (April 2014). "Extrasynaptic NMDA receptor involvement in central nervous system disorders". Neuron. 82 (2): 279–93. doi:10.1016/j.neuron.2014.03.030. PMID 24742457.
- Choi DW, Koh JY, Peters S (January 1988). "Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists". The Journal of Neuroscience. 8 (1): 185–96. doi:10.1523/JNEUROSCI.08-01-00185.1988. PMC 6569373. PMID 2892896.
- Henchcliffe C (2007). Handbook of Clinical Neurology. New York, NY, USA: Weill Medical College of Cornell University, Department of Neurology and Neuroscience. pp. 553–569.
- Hardingham GE, Bading H (February 2003). "The Yin and Yang of NMDA receptor signalling". Trends in Neurosciences. 26 (2): 81–9. doi:10.1016/s0166-2236(02)00040-1. PMID 12536131.
- Hardingham GE, Fukunaga Y, Bading H (May 2002). "Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways". Nature Neuroscience. 5 (5): 405–14. doi:10.1038/nn835. PMID 11953750.
- Xia P, Chen HS, Zhang D, Lipton SA (August 2010). "Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses". The Journal of Neuroscience. 30 (33): 11246–50. doi:10.1523/JNEUROSCI.2488-10.2010. PMC 2932667. PMID 20720132.
- Wang Y, Briz V, Chishti A, Bi X, Baudry M (November 2013). "Distinct roles for μ-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration". The Journal of Neuroscience. 33 (48): 18880–92. doi:10.1523/JNEUROSCI.3293-13.2013. PMC 3841454. PMID 24285894.
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, et al. (July 2009). "Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP". The Journal of Neuroscience. 29 (29): 9330–43. doi:10.1523/JNEUROSCI.2212-09.2009. PMC 2737362. PMID 19625523.
- Karpova A, Mikhaylova M, Bera S, Bär J, Reddy PP, Behnisch T, et al. (February 2013). "Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus". Cell. 152 (5): 1119–33. doi:10.1016/j.cell.2013.02.002. PMID 23452857.
- Berg LK, Larsson M, Morland C, Gundersen V (January 2013). "Pre- and postsynaptic localization of NMDA receptor subunits at hippocampal mossy fibre synapses". Neuroscience. 230: 139–50. doi:10.1016/j.neuroscience.2012.10.061. PMID 23159309.
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ (May 2011). "Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors". The Journal of Neuroscience. 31 (18): 6627–38. doi:10.1523/JNEUROSCI.0203-11.2011. PMC 3100898. PMID 21543591.
- Liu DD, Yang Q, Li ST (April 2013). "Activation of extrasynaptic NMDA receptors induces LTD in rat hippocampal CA1 neurons". Brain Research Bulletin. 93: 10–6. doi:10.1016/j.brainresbull.2012.12.003. PMID 23270879.
- Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. (August 2012). "Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists". Cell. 150 (3): 633–46. doi:10.1016/j.cell.2012.06.029. PMID 22863013.
- Sanz-Clemente A, Nicoll RA, Roche KW (February 2013). "Diversity in NMDA receptor composition: many regulators, many consequences". The Neuroscientist. 19 (1): 62–75. doi:10.1177/1073858411435129. PMC 3567917. PMID 22343826.
- Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, et al. (April 2010). "Organization of NMDA receptors at extrasynaptic locations". Neuroscience. 167 (1): 68–87. doi:10.1016/j.neuroscience.2010.01.022. PMC 2840201. PMID 20096331.
- Lai TW, Shyu WC, Wang YT (May 2011). "Stroke intervention pathways: NMDA receptors and beyond". Trends in Molecular Medicine. 17 (5): 266–75. doi:10.1016/j.molmed.2010.12.008. PMID 21310659.
- Fourie C, Li D, Montgomery JM (February 2014). "The anchoring protein SAP97 influences the trafficking and localisation of multiple membrane channels". Biochimica et Biophysica Acta. 1838 (2): 589–94. doi:10.1016/j.bbamem.2013.03.015. PMID 23535319.
- Lucas DR, Newhouse JP (August 1957). "The toxic effect of sodium L-glutamate on the inner layers of the retina". A.M.A. Archives of Ophthalmology. 58 (2): 193–201. doi:10.1001/archopht.1957.00940010205006. PMID 13443577.
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, et al. (January 2010). "Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice". Neuron. 65 (2): 178–90. doi:10.1016/j.neuron.2010.01.008. PMID 20152125.
- Hardingham GE, Bading H (October 2010). "Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders". Nature Reviews. Neuroscience. 11 (10): 682–96. doi:10.1038/nrn2911. PMC 2948541. PMID 20842175.
- Chen PE, Geballe MT, Stansfeld PJ, Johnston AR, Yuan H, Jacob AL, et al. (May 2005). "Structural features of the glutamate binding site in recombinant NR1/NR2A N-methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling". Molecular Pharmacology. 67 (5): 1470–84. doi:10.1124/mol.104.008185. PMID 15703381.
- Wolosker H (October 2006). "D-serine regulation of NMDA receptor activity". Science's STKE. 2006 (356): pe41. doi:10.1126/stke.3562006pe41. PMID 17033043.
- Yarotskyy V, Glushakov AV, Sumners C, Gravenstein N, Dennis DM, Seubert CN, Martynyuk AE (May 2005). "Differential modulation of glutamatergic transmission by 3,5-dibromo-L-phenylalanine". Molecular Pharmacology. 67 (5): 1648–54. doi:10.1124/mol.104.005983. PMID 15687225.
- Martynyuk AE, Seubert CN, Yarotskyy V, Glushakov AV, Gravenstein N, Sumners C, Dennis DM (November 2006). "Halogenated derivatives of aromatic amino acids exhibit balanced antiglutamatergic actions: potential applications for the treatment of neurological and neuropsychiatric disorders". Recent Patents on CNS Drug Discovery. 1 (3): 261–70. doi:10.2174/157488906778773706. PMID 18221208.
- Cao W, Shah HP, Glushakov AV, Mecca AP, Shi P, Sumners C, et al. (December 2009). "Efficacy of 3,5-dibromo-L-phenylalanine in rat models of stroke, seizures and sensorimotor gating deficit". British Journal of Pharmacology. 158 (8): 2005–13. doi:10.1111/j.1476-5381.2009.00498.x. PMC 2807662. PMID 20050189.
- J. Moskal, D. Leander, R. Burch (2010). Unlocking the Therapeutic Potential of the NMDA Receptor. Drug Discovery & Development News. Retrieved 19 December 2013.
- Anderson C (2003-06-01). "The Bad News Isn't In: A Look at Dissociative-Induced Brain Damage and Cognitive Impairment". Erowid DXM Vaults : Health. Retrieved 2008-12-17.
- Flight MH (December 2013). "Trial watch: phase II boost for glutamate-targeted antidepressants". Nature Reviews. Drug Discovery. 12 (12): 897. doi:10.1038/nrd4178. PMID 24287771.
- Vécsei L, Szalárdy L, Fülöp F, Toldi J (January 2013). "Kynurenines in the CNS: recent advances and new questions". Nature Reviews. Drug Discovery. 12 (1): 64–82. doi:10.1038/nrd3793. PMID 23237916.
- Reis DJ, Regunathan S (May 2000). "Is agmatine a novel neurotransmitter in brain?". Trends in Pharmacological Sciences. 21 (5): 187–93. doi:10.1016/s0165-6147(00)01460-7. PMID 10785653.
- Gibson DA, Harris BR, Rogers DT, Littleton JM (October 2002). "Radioligand binding studies reveal agmatine is a more selective antagonist for a polyamine-site on the NMDA receptor than arcaine or ifenprodil". Brain Research. 952 (1): 71–7. doi:10.1016/s0006-8993(02)03198-0. PMID 12363406.
- Mueller AL, Artman LD, Balandrin MF, Brady E, Chien Y, DelMar EG, et al. (2000). "NPS 1506, a moderate affinity uncompetitive NMDA receptor antagonist: preclinical summary and clinical experience". Amino Acids. 19 (1): 177–9. doi:10.1007/s007260070047. PMID 11026487.
- Monge-Fuentes V, Gomes FM, Campos GA, Silva J, Biolchi AM, Dos Anjos LC, et al. (2015). "Neuroactive compounds obtained from arthropod venoms as new therapeutic platforms for the treatment of neurological disorders". The Journal of Venomous Animals and Toxins Including Tropical Diseases. 21: 31. doi:10.1186/s40409-015-0031-x. PMC 4529710. PMID 26257776.
- Pop E (September 2000). "Nonpsychotropic synthetic cannabinoids". Current Pharmaceutical Design. 6 (13): 1347–60. doi:10.1002/1098-2299(200007/08)50:3/4<211::AID-DDR3>3.0.CO;2-G. PMID 10903397.
- Feigenbaum JJ, Bergmann F, Richmond SA, Mechoulam R, Nadler V, Kloog Y, Sokolovsky M (December 1989). "Nonpsychotropic cannabinoid acts as a functional N-methyl-D-aspartate receptor blocker". Proceedings of the National Academy of Sciences of the United States of America. 86 (23): 9584–7. Bibcode:1989PNAS...86.9584F. doi:10.1073/pnas.86.23.9584. PMC 298542. PMID 2556719.
- Nadler V, Mechoulam R, Sokolovsky M (September 1993). "Blockade of 45Ca2+ influx through the N-methyl-D-aspartate receptor ion channel by the non-psychoactive cannabinoid HU-211". Brain Research. 622 (1–2): 79–85. doi:10.1016/0006-8993(93)90804-v. PMID 8242387.
- Karakas E, Simorowski N, Furukawa H (June 2011). "Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors". Nature. 475 (7355): 249–53. doi:10.1038/nature10180. PMC 3171209. PMID 21677647.
- Glushakov AV, Dennis DM, Morey TE, Sumners C, Cucchiara RF, Seubert CN, Martynyuk AE (2002). "Specific inhibition of N-methyl-D-aspartate receptor function in rat hippocampal neurons by L-phenylalanine at concentrations observed during phenylketonuria". Molecular Psychiatry. 7 (4): 359–67. doi:10.1038/sj.mp.4000976. PMID 11986979.
- Glushakov AV, Glushakova O, Varshney M, Bajpai LK, Sumners C, Laipis PJ, et al. (February 2005). "Long-term changes in glutamatergic synaptic transmission in phenylketonuria". Brain. 128 (Pt 2): 300–7. doi:10.1093/brain/awh354. PMID 15634735.
- Clinical trial number NCT00188383 for "Effects of N-Methyl-D-Aspartate (NMDA)-Receptor Antagonism on Hyperalgesia, Opioid Use, and Pain After Radical Prostatectomy" at ClinicalTrials.gov
- Ludolph AG, Udvardi PT, Schaz U, Henes C, Adolph O, Weigt HU, et al. (May 2010). "Atomoxetine acts as an NMDA receptor blocker in clinically relevant concentrations". British Journal of Pharmacology. 160 (2): 283–91. doi:10.1111/j.1476-5381.2010.00707.x. PMC 2874851. PMID 20423340.
- Shultz RB, Zhong Y (May 2017). "Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury". Neural Regeneration Research. 12 (5): 702–713. doi:10.4103/1673-5374.206633. PMC 5461601. PMID 28616020.
- Lipton SA (October 2007). "Pathologically activated therapeutics for neuroprotection". Nature Reviews. Neuroscience. 8 (10): 803–8. doi:10.1038/nrn2229. PMID 17882256.
- Skolnick P, Boje K, Miller R, Pennington M, Maccecchini ML (October 1992). "Noncompetitive inhibition of N-methyl-D-aspartate by conantokin-G: evidence for an allosteric interaction at polyamine sites". Journal of Neurochemistry. 59 (4): 1516–21. doi:10.1111/j.1471-4159.1992.tb08468.x. PMID 1328523.
- Huggins DJ, Grant GH (January 2005). "The function of the amino terminal domain in NMDA receptor modulation". Journal of Molecular Graphics & Modelling. 23 (4): 381–8. doi:10.1016/j.jmgm.2004.11.006. PMID 15670959.
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. (July 2007). "Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation". Nature Neuroscience. 10 (7): 880–886. doi:10.1038/nn1914. PMC 3910113. PMID 17529984.
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA (January 2008). "Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors". The Journal of Neuroscience. 28 (2): 415–24. doi:10.1523/JNEUROSCI.1900-07.2008. PMC 6670547. PMID 18184784.
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J (September 2005). "Reelin modulates NMDA receptor activity in cortical neurons". The Journal of Neuroscience. 25 (36): 8209–16. doi:10.1523/JNEUROSCI.1951-05.2005. PMC 6725528. PMID 16148228.
- Yu XM, Askalan R, Keil GJ, Salter MW (January 1997). "NMDA channel regulation by channel-associated protein tyrosine kinase Src". Science. 275 (5300): 674–8. doi:10.1126/science.275.5300.674. PMID 9005855.
- Horning MS, Trombley PQ (October 2001). "Zinc and copper influence excitability of rat olfactory bulb neurons by multiple mechanisms". Journal of Neurophysiology. 86 (4): 1652–60. doi:10.1152/jn.2001.86.4.1652. PMID 11600628.
- Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR (July 2010). "Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling". Toxicological Sciences. 116 (1): 249–63. doi:10.1093/toxsci/kfq111. PMC 2886862. PMID 20375082.
- Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer MB, Boulanger LM (December 2010). "MHC class I modulates NMDA receptor function and AMPA receptor trafficking". Proceedings of the National Academy of Sciences of the United States of America. 107 (51): 22278–83. Bibcode:2010PNAS..10722278F. doi:10.1073/pnas.0914064107. PMC 3009822. PMID 21135233.
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ (December 2000). "Functional requirement for class I MHC in CNS development and plasticity". Science. 290 (5499): 2155–9. Bibcode:2000Sci...290.2155H. doi:10.1126/science.290.5499.2155. PMC 2175035. PMID 11118151.
- Nelson PA, Sage JR, Wood SC, Davenport CM, Anagnostaras SG, Boulanger LM (September 2013). "MHC class I immune proteins are critical for hippocampus-dependent memory and gate NMDAR-dependent hippocampal long-term depression". Learning & Memory. 20 (9): 505–17. doi:10.1101/lm.031351.113. PMC 3744042. PMID 23959708.
- Traynelis SF, Cull-Candy SG (May 1990). "Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons". Nature. 345 (6273): 347–50. Bibcode:1990Natur.345..347T. doi:10.1038/345347a0. PMID 1692970.
- Aizenman E, Lipton SA, Loring RH (March 1989). "Selective modulation of NMDA responses by reduction and oxidation". Neuron. 2 (3): 1257–63. doi:10.1016/0896-6273(89)90310-3. PMID 2696504.
- Monaghan DT, Jane DE (2009). "Pharmacology of NMDA Receptors". In Van Dongen AM (ed.). Biology of the NMDA Receptor. Boca Raton, Florida: CRC Press. ISBN 978-1-4200-4414-0.
- Sonkusare SK, Kaul CL, Ramarao P (January 2005). "Dementia of Alzheimer's disease and other neurodegenerative disorders--memantine, a new hope". Pharmacological Research. 51 (1): 1–17. doi:10.1016/j.phrs.2004.05.005. PMID 15519530.
- Takahashi H, Xia P, Cui J, Talantova M, Bodhinathan K, Li W, et al. (October 2015). "Pharmacologically targeted NMDA receptor antagonism by NitroMemantine for cerebrovascular disease". Scientific Reports. 5: 14781. Bibcode:2015NatSR...514781T. doi:10.1038/srep14781. PMC 4609936. PMID 26477507.
- Purves, Dale; George J. Augustine; David Fitzpatrick; William C. Hall; Anthony-Samuel LaMantia; James O. McNamara; Leonard E. White (2008). Neuroscience, 4th Ed. Sinauer Associates. pp. 129–131. ISBN 978-0-87893-697-7. Archived from the original on 2011-09-27.
- Vargas-Caballero M, Robinson HP (July 2004). "Fast and slow voltage-dependent dynamics of magnesium block in the NMDA receptor: the asymmetric trapping block model". The Journal of Neuroscience. 24 (27): 6171–80. doi:10.1523/jneurosci.1380-04.2004. PMC 6729657. PMID 15240809.
- Purves, Dale; George J. Augustine; David Fitzpatrick; William C. Hall; Anthony-Samuel LaMantia; James O. McNamara; Leonard E. White (2008). Neuroscience, 4th Ed. Sinauer Associates. pp. 191–195. ISBN 978-0-87893-697-7. Archived from the original on 2011-09-27.
- Wijesinghe R (2014). "Emerging Therapies for Treatment Resistant Depression". Ment Health Clin. 4 (5): 56. doi:10.9740/mhc.n207179. ISSN 2168-9709.
- Poon L (2014). "Growing Evidence That A Party Drug Can Help Severe Depression". NPR.
- Stix G (2014). "From Club to Clinic: Physicians Push Off-Label Ketamine as Rapid Depression Treatment". Scientific American.
- McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, Fuchs E (March 2010). "The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation". Molecular Psychiatry. 15 (3): 237–49. doi:10.1038/mp.2009.80. PMC 2902200. PMID 19704408.
- McEwen BS, Chattarji S (December 2004). "Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine". European Neuropsychopharmacology. 14 Suppl 5: S497–502. doi:10.1016/j.euroneuro.2004.09.008. PMID 15550348.
- McEwen BS, Olié JP (June 2005). "Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine". Molecular Psychiatry. 10 (6): 525–37. doi:10.1038/sj.mp.4001648. PMID 15753957.
- Brink CB, Harvey BH, Brand L (January 2006). "Tianeptine: a novel atypical antidepressant that may provide new insights into the biomolecular basis of depression". Recent Patents on CNS Drug Discovery. 1 (1): 29–41. doi:10.2174/157488906775245327. PMID 18221189.
- Kasper S, McEwen BS (2008). "Neurobiological and clinical effects of the antidepressant tianeptine". CNS Drugs. 22 (1): 15–26. doi:10.2165/00023210-200822010-00002. PMID 18072812.
- Mount C, Downton C (July 2006). "Alzheimer disease: progress or profit?". Nature Medicine. 12 (7): 780–4. doi:10.1038/nm0706-780. PMID 16829947.
- NICE technology appraisal January 18, 2011 Azheimer's disease - donepezil, galantamine, rivastigmine and memantine (review): final appraisal determination
- Todd A Hardy, Reddel, Barnett, Palace, Lucchinetti, Weinshenker, Atypical inflammatory demyelinating syndromes of the CNS, The lancet neurology, Volume 15, Issue 9, August 2016, Pages 967-981, doi: https://doi.org/10.1016/S1474-4422(16)30043-6, available at
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA (May 2008). "Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia". Trends in Neurosciences. 31 (5): 234–42. doi:10.1016/j.tins.2008.02.005. PMC 2680493. PMID 18395805.
External links

- NMDA receptor pharmacology
- Motor Discoordination Results from Combined Gene Disruption of the NMDA Receptor NR2A and NR2C Subunits, But Not from Single Disruption of the NR2A or NR2C Subunit
- A schematic diagram summarizes three potential models for the switching of NR2A and NR2B subunits at developing synapses
- Drosophila NMDA receptor 1 - The Interactive Fly