Dimethyl methylphosphonate
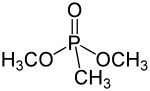
Dimethyl methylphosphonate is an organophosphorus compound with the chemical formula CH3PO(OCH3)2. It is a colourless liquid which is primarily used as a flame retardant.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(methoxy-methylphosphoryl)oxymethane | |
Other names
| |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.957 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C3H9O3P |
| Molar mass | 124.076 g·mol−1 |
| Appearance | colourless liquid |
| Density | 1.145 g/mL at 25 °C |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 181 °C (358 °F; 454 K) |
Solubility in water |
slowly hydrolyses |
| Hazards | |
| Main hazards | Toxic |
| R-phrases (outdated) | R46, R36 |
| Flash point | 69 °C (156 °F; 342 K) closed cup |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
Dimethyl methylphosphonate can be prepared from trimethyl phosphite and a halomethane (e.g. iodomethane) via the Michaelis–Arbuzov reaction.
Dimethyl methylphosphonate is a schedule 2 chemical as it may be used in the production of chemical weapons. It will react with thionyl chloride to produce methylphosphonic acid dichloride, which is used in the production of sarin and soman nerve agents. Various amines can be used to catalyse this process.[1] It can be used as a sarin-simulant for the calibration of organophosphorus detectors.
Uses
The primary commercial use of dimethyl methylphosphonate is as a flame retardant. Other commercial uses are a preignition additive for gasoline, anti-foaming agent, plasticizer, stabilizer, textile conditioner, antistatic agent, and an additive for solvents and low-temperature hydraulic fluids. It can be used as a catalyst and a reagent in organic synthesis, as it can generate a highly reactive ylide. The yearly production in the United States varies between 100 and 1,000 short tons (91,000 and 907,000 kg).
About 190 liters of dimethyl methylphosphonate, together with other chemicals, were released during the crash of El Al Flight 1862 at Bijlmer, Amsterdam in 1992.
References
- Maier, Ludwig (1990). "ORGANIC PHOSPHORUS COMPOUNDS 90.l A CONVENIENT, ONE-STEP SYNTHESIS OF ALKYL- AND ARYLPHOSPHONYL DICHLORIDES". Phosphorus, Sulfur, and Silicon and the Related Elements. 47 (3–4): 465–470. doi:10.1080/10426509008038002.