Diabetic nephropathy
Diabetic nephropathy (DN), also known as diabetic kidney disease,[4] is the chronic loss of kidney function occurring in those with diabetes mellitus. Protein loss in the urine due to damage to the glomeruli may become massive, and cause a low serum albumin with resulting generalized body swelling (edema) and result in the nephrotic syndrome. Likewise, the estimated glomerular filtration rate (eGFR) may progressively fall from a normal of over 90 ml/min/1.73m2 to less than 15, at which point the patient is said to have end-stage kidney disease (ESKD).[5] It usually is slowly progressive over years.[6]
| Diabetic nephropathy | |
|---|---|
| Other names | Diabetic kidney disease |
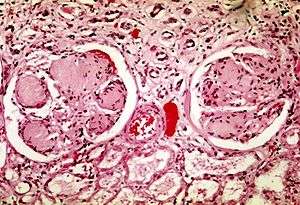 | |
| Two glomeruli in diabetic nephropathy: the acellular light purple areas within the capillary tufts are the destructive mesangial matrix deposits. | |
| Specialty | Endocrinology |
| Risk factors | High blood pressure, Unstable blood glucose[1] |
| Diagnostic method | Abnormal levels of urinary albumin[2] |
| Treatment | ACE inhibitors[3] |
Pathophysiologic abnormalities in DN begin with long-standing poorly controlled blood glucose levels. This is followed by multiple changes in the filtration units of the kidneys, the nephrons. (There are normally about 750,000–1.5 million nephrons in each adult kidney).[7] Initially, there is constriction of the efferent arterioles and dilation of afferent arterioles, with resulting glomerular capillary hypertension and hyperfiltration; this gradually changes to hypofiltration over time.[8] Concurrently, there are changes within the glomerulus itself: these include a thickening of the basement membrane, a widening of the slit membranes of the podocytes, an increase in the number of mesangial cells, and an increase in mesangial matrix. This matrix invades the glomerular capillaries and produces deposits called Kimmelstiel-Wilson nodules. The mesangial cells and matrix can progressively expand and consume the entire glomerulus, shutting off filtration.[9]
The status of DN may be monitored by measuring two values: the amount of protein in the urine - proteinuria; and a blood test called the serum creatinine. The amount of the proteinuria reflects the degree of damage to any still-functioning glomeruli. The value of the serum creatinine can be used to calculate the estimated glomerular filtration rate (eGFR), which reflects the percentage of glomeruli which are no longer filtering the blood. Treatment with an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), which dilates the arteriole exiting the glomerulus, thus reducing the blood pressure within the glomerular capillaries, which may slow (but not stop) progression of the disease. Three classes of diabetes medications – GLP-1 agonists, DPP-4 inhibitors, and SGLT2 inhibitors – are also thought to slow the progression of diabetic nephropathy.[10]
Diabetic nephropathy is the most common cause of ESKD and is a serious complication that affects approximately one quarter of adults with diabetes in the United States.[11][12] Affected individuals with end-stage kidney disease often require hemodialysis and eventually kidney transplantation to replace the failed kidney function.[13] Diabetic nephropathy is associated with an increased risk of death in general, particularly from cardiovascular disease.[11][14]
Signs and symptoms
The onset of symptoms is 5 to 10 years after the disease begins.[1] A usual first symptom is frequent urination at night: nocturia. Other symptoms include tiredness, headaches, a general feeling of illness, nausea, vomiting, frequent daytime urination, lack of appetite, itchy skin, and leg swelling.[1]
Risk factors
The incidence of diabetic nephropathy is higher in people with diabetes that have one or more of the following conditions:[1]
- Poor control of blood glucose
- Uncontrolled high blood pressure
- Type 1 diabetes mellitus, with onset before age 20
- Past or current cigarette use
- A family history of diabetic nephropathy
Pathophysiology
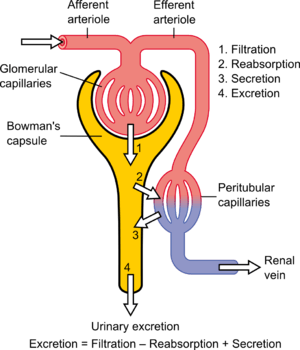
The pathophysiology of the glomerulus in DN can best be understood by considering the three involved cells as a unit: the endothelial cell, the podocyte, and the mesangial cell. These cells are in physical contact with one another at various locations within the glomerulus; they also communicate with one another chemically at a distance. All three cells are abnormal in DN.[9]
Diabetes causes a number of changes to the body's metabolism and blood circulation, which likely combine to produce excess reactive oxygen species (chemically reactive molecules containing oxygen). These changes damage the kidney's glomeruli (networks of tiny blood vessels), which leads to the hallmark feature of albumin in the urine (called albuminuria).[15] As diabetic nephropathy progresses, a structure in the glomeruli known as the glomerular filtration barrier (GFB) is increasingly damaged.[11] This barrier is composed of three layers including the fenestrated endothelium, the glomerular basement membrane, and the epithelial podocytes.[11] The GFB is responsible for the highly selective filtration of blood entering the kidney's glomeruli and normally only allows the passage of water, small molecules, and very small proteins (albumin does not pass through the intact GFB).[11] Damage to the glomerular basement membrane allows proteins in the blood to leak through, leading to proteinuria. Deposition of abnormally large amounts of mesangial matrix causes periodic-acid schiff positive nodules called Kimmelstiel–Wilson nodules.[16]
High blood sugar, which leads to formation of advanced glycation end products; and cytokines have also been implicated as mechanisms for the development of diabetic nephropathy.[17]
Diagnosis
Diagnosis is based on the measurement of abnormal levels of urinary albumin in a diabetic[2] coupled with exclusion of other causes of albuminuria. Albumin measurements are defined as follows:[18]
- Normal albuminuria: urinary albumin excretion <30 mg/24h;
- Microalbuminuria: urinary albumin excretion in the range of 30–299 mg/24h;
- Macroalbuminuria: urinary albumin excretion ≥300 mg/24h.
It is recommended that diabetics have their albumin levels checked annually, beginning immediately after a diagnosis of type 2 diabetes and five years after a diagnosis of type 1 diabetes.[2][19]
Medical imaging of the kidneys, generally by ultrasonography, is recommended as part of a differential diagnosis if there is suspicion of urinary tract obstruction, urinary tract infection, or kidney stones or polycystic kidney disease.[20]
| CKD Stage[21] | eGFR level (mL/min/1.73 m2) |
|---|---|
| Stage 1 | ≥ 90 |
| Stage 2 | 60–89 |
| Stage 3 | 30–59 |
| Stage 4 | 15–29 |
| Stage 5 | < 15 |
Treatment
The goals of treatment are to slow the progression of kidney damage and control related complications. The main treatment, once proteinuria is established, is ACE inhibitor medications, which usually reduce proteinuria levels and slow the progression of diabetic nephropathy.[3] Other issues that are important in the management of this condition include control of high blood pressure and blood sugar levels (see diabetes management), as well as the reduction of dietary salt intake.[23]
Prognosis
Diabetic nephropathy in type 2 diabetes can be more difficult to predict because the onset of diabetes is not usually well established. Without intervention, 20–40 percent of patients with type 2 diabetes/microalbuminuria, will evolve to macroalbuminuria.[24]
Diabetic nephropathy is the most common cause of end-stage kidney disease,[11][12] which may require hemodialysis or even kidney transplantation.[13] It is associated with an increased risk of death in general, particularly from cardiovascular disease.[11][14]
Epidemiology
In the U.S., diabetic nephropathy affected an estimated 6.9 million people during 2005–2008.[25] The number of people with diabetes and consequently diabetic nephropathy is expected to rise substantially by the year 2050.[26]
See also
References
- "Diabetes and kidney disease: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2015-06-27.
- Lewis G, Maxwell AP (February 2014). "Risk factor control is key in diabetic nephropathy". The Practitioner. 258 (1768): 13–7, 2. PMID 24689163.
- Lim AK (2014). "Diabetic nephropathy – complications and treatment". International Journal of Nephrology and Renovascular Disease. 7: 361–81. doi:10.2147/IJNRD.S40172. PMC 4206379. PMID 25342915.
- Kittell F (2012). "Diabetes Management". In Thomas LK, Othersen JB (eds.). Nutrition Therapy for Chronic Kidney Disease. CRC Press. p. 198.
- Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J (2013). Harrison's manual of medicine (18th ed.). New York: McGraw-Hill Medical. p. 2982. ISBN 978-0-07-174519-2.
- Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH (August 2016). "Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988–2014". JAMA. 316 (6): 602–10. doi:10.1001/jama.2016.10924. PMC 5444809. PMID 27532915.
- Hall J, Guyton A (2005). Textbook of Medical Physiology (11th ed.). Philadelphia: W.B. Saunders. p. 310. ISBN 978-0-7216-0240-0.
- "diabetic nephropathy". Retrieved 2015-06-27.
- Schlöndorff D, Banas B (June 2009). "The mesangial cell revisited: no cell is an island". Journal of the American Society of Nephrology. 20 (6): 1179–87. doi:10.1681/ASN.2008050549. PMID 19470685.
- de Boer IH (August 2017). "A New Chapter for Diabetic Kidney Disease". The New England Journal of Medicine. 377 (9): 885–887. doi:10.1056/nejme1708949. PMID 28854097.
- Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, Navarro-González JF (September 2014). "Diabetic kidney disease: from physiology to therapeutics". The Journal of Physiology. 592 (18): 3997–4012. doi:10.1113/jphysiol.2014.272328. PMC 4198010. PMID 24907306.
- Ding Y, Choi ME (January 2015). "Autophagy in diabetic nephropathy". The Journal of Endocrinology. 224 (1): R15–30. doi:10.1530/JOE-14-0437. PMC 4238413. PMID 25349246.
- Lizicarova D, Krahulec B, Hirnerova E, Gaspar L, Celecova Z (2014). "Risk factors in diabetic nephropathy progression at present". Bratislavske Lekarske Listy. 115 (8): 517–21. doi:10.4149/BLL_2014_101. PMID 25246291.
- Pálsson R, Patel UD (May 2014). "Cardiovascular complications of diabetic kidney disease". Advances in Chronic Kidney Disease. 21 (3): 273–80. doi:10.1053/j.ackd.2014.03.003. PMC 4045477. PMID 24780455.
- Cao Z, Cooper ME (August 2011). "Pathogenesis of diabetic nephropathy". Journal of Diabetes Investigation. 2 (4): 243–7. doi:10.1111/j.2040-1124.2011.00131.x. PMC 4014960. PMID 24843491.
- Kimmelstiel, Paul; Wilson, Clifford (1936). "Intercapillary lesions in the glomeruli of the kidney". The American Journal of Pathology. 12 (1): 83–98.7. PMC 1911022. PMID 19970254.
- "Diabetic Nephropathy: Background, Pathophysiology, Etiology". 2015-06-20. Cite journal requires
|journal=(help) - "CDC – Chronic Kidney Disease – Glossary". Retrieved 2015-07-02.
- Koroshi A (July 2007). "Microalbuminuria, is it so important?". Hippokratia. 11 (3): 105–7. PMC 2658722. PMID 19582202.
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (January 2005). "Diabetic nephropathy: diagnosis, prevention, and treatment". Diabetes Care. 28 (1): 164–76. doi:10.2337/diacare.28.1.164. PMID 15616252.
- Fink HA, Ishani A, Taylor BC, Greer NL, MacDonald R, Rossini D, et al. (January 2012). Chronic Kidney Disease Stages 1–3: Screening, Monitoring, and Treatment [Internet]. Agency for Healthcare Research and Quality (US). PMID 22439155.
- "Glomerular filtration rate: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2015-07-02.
- "Diabetic Nephropathy Treatment & Management: Approach Considerations, Glycemic Control, Management of Hypertension". 2015-06-20. Cite journal requires
|journal=(help) - Shlipak M (2011-03-15). "Clinical Evidence Handbook: Diabetic Nephropathy: Preventing Progression – American Family Physician". American Family Physician. 83 (6): 732. Retrieved 2015-06-27.
- Lerma EV (2014-01-01). Diabetes and Kidney Disease. Springer. ISBN 9781493907939.
- Lai KN, Tang SC (2011-06-08). Diabetes and the Kidney. Karger Medical and Scientific Publishers. ISBN 9783805597432.
Further reading
- "Effects of renin-angiotensin system blockers on renal outcomes and all-cause mortality in patients with diabetic nephropathy: an updated meta-analysis". www.crd.york.ac.uk. Retrieved 2015-07-02.
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (January 2005). "Diabetic nephropathy: diagnosis, prevention, and treatment". Diabetes Care. 28 (1): 164–76. doi:10.2337/diacare.28.1.164. PMID 15616252.
- Tziomalos K, Athyros VG (2015). "Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis". The Review of Diabetic Studies. 12 (1–2): 110–8. doi:10.1900/RDS.2015.12.110. PMC 5397986. PMID 26676664.
- Kume S, Koya D, Uzu T, Maegawa H (2014). "Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy". BioMed Research International. 2014: 315494. doi:10.1155/2014/315494. PMC 4122096. PMID 25126552.
- Doshi SM, Friedman AN (August 2017). "Diagnosis and Management of Type 2 Diabetic Kidney Disease". Clinical Journal of the American Society of Nephrology. 12 (8): 1366–1373. doi:10.2215/CJN.11111016. PMC 5544517. PMID 28280116.
External links
| Classification | |
|---|---|
| External resources |