Detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties in dilute solutions.[1] These substances are usually alkylbenzenesulfonates, a family of compounds that are similar to soap but are more soluble in hard water, because the polar sulfonate (of detergents) is less likely than the polar carboxylate (of soap) to bind to calcium and other ions found in hard water.
In house contexts, the term detergent by itself refers specifically to laundry detergent or dish detergent, as opposed to hand soap or other types of cleaning agents. Detergents are commonly available as powders or concentrated solutions. Detergents, like soaps, work because they are amphiphilic: partly hydrophilic (polar) and partly hydrophobic (non-polar). Their dual nature facilitates the mixture of hydrophobic compounds (like oil and grease) with water. Because air is not hydrophilic, detergents are also foaming agents to varying degrees.
Chemical classifications of detergents
Detergents are classified into three broad groupings, depending on the electrical charge of the surfactants.
Anionic detergents

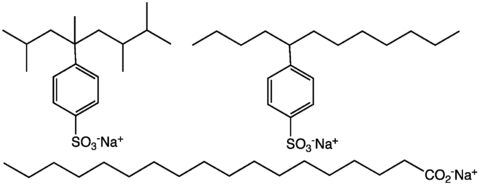
Typical anionic detergents are alkylbenzenesulfonates. The alkylbenzene portion of these anions is lipophilic and the sulfonate is hydrophilic. Two different varieties have been popularized, those with branched alkyl groups and those with linear alkyl groups. The former were largely phased out in economically advanced societies because they are poorly biodegradable.[2] An estimated 6 billion kilograms of anionic detergents are produced annually for domestic markets.
Bile acids, such as deoxycholic acid (DOC), are anionic detergents produced by the liver to aid in digestion and absorption of fats and oils.
Cationic detergents
Cationic detergents that are similar to the anionic ones, with a hydrophilic component, but, instead of the anionic sulfonate group, the cationic surfactants have quaternary ammonium as the polar end. The ammonium sulfate center is positively charged.[2]
Non-ionic and zwitter ionic detergents
Non-ionic detergents are characterized by their uncharged, hydrophilic headgroups. Typical non-ionic detergents are based on polyoxyethylene or a glycoside. Common examples of the former include Tween, Triton, and the Brij series. These materials are also known as ethoxylates or PEGylates and their metabolites, nonylphenol. Glycosides have a sugar as their uncharged hydrophilic headgroup. Examples include octyl thioglucoside and maltosides. HEGA and MEGA series detergents are similar, possessing a sugar alcohol as headgroup.
Zwitterionic detergents possess a net zero charge arising from the presence of equal numbers of +1 and −1 charged chemical groups. Examples include CHAPS.
See surfactants for more applications.
History
In World War I, there was a shortage of oils. Synthetic detergents were first made in Germany.[3][4]
Major applications of detergents
Household cleaning
One of the largest applications of detergents is for household and shop cleaning including dish washing and washing laundry. The formulations are complex, reflecting the diverse demands of the application and the highly competitive consumer market.
Fuel additives
Both carburetors and fuel injector components of Otto engines benefit from detergents in the fuels to prevent fouling. Concentrations are about 300 ppm. Typical detergents are long-chain amines and amides such as polyisobuteneamine and polyisobuteneamide/succinimide.[5]
Biological reagent
Reagent grade detergents are employed for the isolation and purification of integral membrane proteins found in biological cells.[6] Solubilization of cell membrane bilayers requires a detergent that can enter the inner membrane monolayer.[7] Advancements in the purity and sophistication of detergents have facilitated structural and biophysical characterization of important membrane proteins such as ion channels also the disrupt membrane by binding lipopolysaccharide,[8] transporters, signaling receptors, and photosystem II.[9]
See also
- Soap
- Cleavable detergent
- Dishwashing liquid
- Dispersant
- Green cleaning
- Hard-surface cleaner
- Laundry detergent
- List of cleaning products
- Triton X-100
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "detergent". doi:10.1351/goldbook.D01643
- Eduard Smulders, Wolfgang Rybinski, Eric Sung, Wilfried Rähse, Josef Steber, Frederike Wiebel, Anette Nordskog, "Laundry Detergents" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_315.pub2
- "Soaps & Detergent: History (1900s to Now)". American Cleaning Institute. Retrieved on 6 January 2015
- David O. Whitten; Bessie Emrick Whitten (1 January 1997). Handbook of American Business History: Extractives, manufacturing, and services. Greenwood Publishing Group. p. 221. ISBN 978-0-313-25199-3 – via Google Books.
- Werner Dabelstein, Arno Reglitzky, Andrea Schütze, Klaus Reders "Automotive Fuels" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheimdoi:10.1002/14356007.a16_719.pub2
- Koley D, Bard AJ (2010). "Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM)". Proceedings of the National Academy of Sciences of the United States of America. 107 (39): 16783–7. Bibcode:2010PNAS..10716783K. doi:10.1073/pnas.1011614107. PMC 2947864. PMID 20837548.
- Lichtenberg D, Ahyayauch H, Goñi FM (2013). "The mechanism of detergent solubilization of lipid bilayers". Biophysical Journal. 105 (2): 289–299. Bibcode:2013BpJ...105..289L. doi:10.1016/j.bpj.2013.06.007. PMC 3714928. PMID 23870250.
- Doyle, DA; Morais Cabral, J; Pfuetzner, RA; Kuo, A; Gulbis, JM; Cohen, SL; Chait, BT; MacKinnon, R (1998). "The structure of the potassium channel: molecular basis of K+conduction and selectivity". Science. 280 (5360): 69–77. Bibcode:1998Sci...280...69D. doi:10.1126/science.280.5360.69. PMID 9525859.
- Umena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren; Kamiya, Nobuo (2011). "Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 A" (PDF). Nature. 473 (7345): 55–60. Bibcode:2011Natur.473...55U. doi:10.1038/nature09913. PMID 21499260.
External links
| Wikimedia Commons has media related to Detergents. |
- About.com: How Do Detergents Clean
- Campbell tips for detergents chemistry, surfactants, and history related to laundry washing, destaining methods and soil.