Calculus (dental)
In dentistry, calculus or tartar is a form of hardened dental plaque. It is caused by precipitation of minerals from saliva and gingival crevicular fluid (GCF) in plaque on the teeth. This process of precipitation kills the bacterial cells within dental plaque, but the rough and hardened surface that is formed provides an ideal surface for further plaque formation. This leads to calculus buildup, which compromises the health of the gingiva (gums). Calculus can form both along the gumline, where it is referred to as supragingival ("above the gum"), and within the narrow sulcus that exists between the teeth and the gingiva, where it is referred to as subgingival ("below the gum").
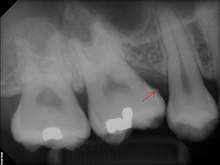
Calculus formation is associated with a number of clinical manifestations, including bad breath, receding gums and chronically inflamed gingiva. Brushing and flossing can remove plaque from which calculus forms; however, once formed, calculus is too hard (firmly attached) to be removed with a toothbrush. Calculus buildup can be removed with ultrasonic tools or dental hand instruments (such as a periodontal scaler).
Etymology
The word comes from Latin calculus "small stone", from calx "limestone, lime",[1] probably related to Greek χάλιξ chalix "small stone, pebble, rubble,"[2] which many trace to a Proto-Indo-European root for "split, break up".[3] Calculus was a term used for various kinds of stones. This spun off many modern words, including "calculate" (use stones for mathematical purposes), and "calculus", which came to be used, in the 18th century, for accidental or incidental mineral buildups in human and animal bodies, like kidney stones and minerals on teeth.[3]
Tartar, on the other hand, originates in Greek as well (tartaron), but as the term for the white encrustation inside casks, aka potassium bitartrate commonly known as cream of tartar. This came to be a term used for calcium phosphate on teeth in the early 19th century.[4]
Calculus composition
Calculus is composed of both inorganic (mineral) and organic (cellular and extracellular matrix) components. The mineral proportion of calculus ranges from approximately 40–60%, depending on its location in the dentition,[5] and consists primarily of calcium phosphate crystals organized into four principal mineral phases, listed here in order of decreasing ratio of phosphate to calcium:
- hydroxyapatite, Ca
5(PO
4)
3OH, - whitlockite, Ca9(Mg,Fe)(PO4)6(PO3OH),
- octacalcium phosphate, Ca8H2(PO4)6.5H2O,
- and brushite, CaHPO
4·2H
2O.
The organic component of calculus is approximately 85% cellular and 15% extracellular matrix.[5] Cell density within dental plaque and calculus is very high, consisting of an estimated 200,000,000 cells per milligram.[6][7] The cells within calculus are primarily bacterial, but also include at least one species of archaea (Methanobrevibacter oralis) and several species of yeast (e.g., Candida albicans). The organic extracellular matrix in calculus consists primarily of proteins and lipids (fatty acids, triglycerides, glycolipids, and phospholipids),[5] as well as extracellular DNA.[6][8] Trace amounts of host, dietary, and environmental microdebris are also found within calculus, including salivary proteins,[9] plant DNA,[10] milk proteins,[11] starch granules,[12] textile fibers,[13] and smoke particles.[14]
Calculus formation
The processes of calculus formation from dental plaque are not well understood. Supragingival calculus formation is most abundant on the buccal (cheek) surfaces of the maxillary (upper jaw) molars and on the lingual (tongue) surfaces of the mandibular (lower jaw) incisors.[15] These areas experience high salivary flow because of their proximity to the parotid and sublingual salivary glands. Subgingival calculus forms below the gumline and is typically darkened in color by the presence of black-pigmented bacteria,[15] whose cells are coated in a layer of iron obtained from heme during gingival bleeding.[16] Dental calculus typically forms in incremental layers[17] that are easily visible using both electron microscopy and light microscopy.[9] These layers form during periodic calcification events of the dental plaque,[15] but the timing and triggers of these events are poorly understood. The formation of calculus varies widely among individuals and at different locations within the mouth. Many variables have been identified that influence the formation of dental calculus, including age, gender, ethnic background, diet, location in the oral cavity, oral hygiene, bacterial plaque composition, host genetics, access to professional dental care, physical disabilities, systemic diseases, tobacco use, and drugs and medications.[15]
Clinical significance
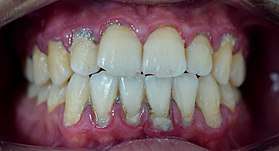
Plaque accumulation causes the gingiva to become irritated and inflamed, and this is referred to as gingivitis. When the gingiva become so irritated that there is a loss of the connective tissue fibers that attach the gums to the teeth and bone that surrounds the tooth, this is known as periodontitis. Dental plaque is not the sole cause of periodontitis; however it is many times referred to as a primary aetiology. Plaque that remains in the oral cavity long enough will eventually calcify and become calculus.[15] Calculus is detrimental to gingival health because it serves as a trap for increased plaque formation and retention; thus, calculus, along with other factors that cause a localized build-up of plaque, is referred to as a secondary aetiology of periodontitis.
When plaque is supragingival, the bacterial content contains a great proportion of aerobic bacteria and yeast,[18] or those bacteria which utilize and can survive in an environment containing oxygen. Subgingival plaque contains a higher proportion of anaerobic bacteria, or those bacteria which cannot exist in an environment containing oxygen. Several anaerobic plaque bacteria, such as Porphyromonas gingivalis,[19] secrete antigenic proteins that trigger a strong inflammatory response in the periodontium, the specialized tissues that surround and support the teeth. Prolonged inflammation of the periodontium leads to bone loss and weakening of the gingival fibers that attach the teeth to the gums, two major hallmarks of periodontitis. Supragingival calculus formation is nearly ubiquitous in humans,[20][21][22] but to differing degrees. Almost all individuals with periodontitis exhibit considerable subgingival calculus deposits.[15] Dental plaque bacteria have been linked to cardiovascular disease[23] and mothers giving birth to pre-term low weight infants,[24] but there is no conclusive evidence yet that periodontitis is a significant risk factor for either of these two conditions.[25]
Prevention
Toothpaste with zinc citrate has been shown to produce a statistically significant reduction in plaque accumulation, but it is so modest that its clinical importance is questionable.[26] Some calculus may form even without plaque deposits, by direct mineralisation of the pellicle.
Calculus in animals
Calculus formation in other animals is less well studied than in humans, but it is known to form in a wide range of species. Domestic pets, such as dogs and cats, frequently accumulate large calculus deposits.[27] Animals with highly abrasive diets, such as ruminants and equids, rarely form thick deposits and instead tend to form thin calculus deposits that often have a metallic or opalescent sheen.[28] In animals, calculus should not be confused with crown cementum,[29] a layer of calcified dental tissue that encases the tooth root underneath the gingival margin and is gradually lost through periodontal disease.
Archaeological significance
Dental calculus has been shown to contain well preserved microparticles, DNA and protein in archaeological samples.[30] The information these molecules contain can reveal information about the oral microbiome of the host and the presence of pathogens.[31] It is also possible to identify dietary sources[32] as well as study dietary shifts[33] and occasionally evidence of craft activities.[34]
Sub-gingival calculus formation and chemical dissolution
Sub-gingival calculus is composed almost entirely of two components: fossilized anaerobic bacteria whose biological composition has been replaced by calcium phosphate salts, and calcium phosphate salts that have joined the fossilized bacteria in calculus formations. The initial attachment mechanism and the development of mature calculus formations are based on electrical charge. Unlike calcium phosphate, the primary component of teeth, calcium phosphate salts exist as electrically unstable ions. The following minerals are detectable in calculus by X-ray diffraction: brushite (CaHPO4·2H2O), octacalcium phosphate (Ca8H2(PO4)6.5H2O), magnesium-containing whitlockite (Ca9(MgFe)(PO4)6PO3OH), and carbonate-containing hydroxyapatite (approximately Ca5(PO4)3(OH) but containing some carbonate).[35]
The reason fossilized bacteria are initially attracted to one part of the subgingival tooth surface over another is not fully understood; once the first layer is attached, ionized calculus components are naturally attracted to the same places due to electrical charge. The fossilized bacteria pile on top of one another, in a rather haphazard manner. All the while, free-floating ionic components fill in the gaps left by the fossilized bacteria. The resultant hardened structure can be compared to concrete; with the fossilized bacteria playing the role of aggregate, and the smaller calcium phosphate salts being the cement. The once purely electrical association of fossilized bacteria then becomes mechanical, with the introduction of free-floating calcium phosphate salts. The "hardened" calculus formations are at the heart of periodontal disease and treatment.
Removal of calculus after formation
The College of Registered Dental Hygienists of Alberta (CRDHA) defines a dental hygienist as "a health care professional whose work focuses on the oral health of an individual or community."[36] These dental professionals aim to improve oral health by educating patients on the prevention and management of oral disease.[36] Dental hygienists can be found performing oral health services in various settings, including private dental offices, schools, and other community settings, such as long-term care facilities.[37] As mentioned above in the clinical significance section, plaque and calculus deposits are a major etiological factor in the development and progression of oral disease. An important part of the scope of practice of a dental hygienist is the removal of plaque and calculus deposits. This is achieved through the use of specifically designed instruments for debridement of tooth surfaces.[38][39] Treatment with these types of instruments is necessary as calculus deposits cannot be removed by brushing or flossing alone. To effectively manage disease or maintain oral health, thorough removal of calculus deposits should be completed at frequent intervals. The recommended frequency of dental hygiene treatment can be made by a registered professional, and is dependent on individual patient needs.[40] Factors that are taken into consideration include an individual's overall health status, tobacco use, amount of calculus present, and adherence to a professionally recommended home care routine.[41]
Hand instruments are specially designed tools used by dental professionals to remove plaque and calculus deposits that have formed on the teeth.[38][39] These tools include scalers, curettes, jaquettes, hoes, files and chisels.[38][39] Each type of tool is designed to be used in specific areas of the mouth.[39] Some commonly used instruments include sickle scalers which are designed with a pointed tip and are mainly used supragingivally.[38][39] Curettes are mainly used to remove subgingival calculus, smooth root surfaces and to clean out periodontal pockets.[38][42] Curettes can be divided into two subgroups: universals and area specific instruments. Universal curettes can be used in multiple areas, while area specific instruments are designed for select tooth surfaces.[39] Gracey curettes are a popular type of area specific curettes.[39] Due to their design, area specific curettes allow for better adaptation to the root surface and can be slightly more effective than universals.[38][39] Hoes, chisels, and files are less widely used than scalers and curettes. These are beneficial when removing large amounts of calculus or tenacious calculus that cannot be removed with a curette or scaler alone.[38] Chisels and hoes are used to remove bands of calculus, whereas files are used to crush burnished or tenacious calculus.[38]
For hand instrumentation to be effective and efficient, it is important for clinicians to ensure that the instruments being used are sharp.[38][39] It is also important for the clinician to understand the design of the hand instruments to be able to adapt them properly.[38]
Ultrasonic scalers, also known as power scalers, are effective in removing calculus, stain, and plaque. These scalers are also useful for root planing, curettage, and surgical debridement.[38] Not only is tenacious calculus and stain removed more effectively with ultrasonic scalers than with hand instrumentation alone, it is evident that the most satisfactory clinical results are when ultrasonics are used in adjunct to hand instrumentation.[38] There are two types of ultrasonic scalers; piezoelectric and magnetostrictive. Oscillating material in both of these handpieces cause the tip of the scaler to vibrate at high speeds, between 18,000 and 50,000 Hz.[38] The tip of each scaler uses a different vibration pattern for removal of calculus.[38] The magnetostrictive power scaler vibration is elliptical, activating all sides of the tip, whereas the piezoelectric vibration is linear and is more active on the two sides of the tip.[38]
Special tips for ultrasonic scalers are designed to address different areas of the mouth and varying amounts of calculus buildup. Larger tips are used for heavy subgingival or supragingival calculus deposits, whereas thinner tips are designed more for definitive subgingival debridement.[38] As the high frequency vibrations loosen calculus and plaque, heat is generated at the tip.[38] A water spray is directed towards the end of the tip to cool it as well as irrigate the gingiva during debridement.[38] Only the first 1–2 mm of the tip on the ultrasonic scaler is most effective for removal, and therefore needs to come into direct contact with the calculus to fracture the deposits.[38] Small adaptations are needed in order to keep the tip of the scaler touching the surface of the tooth, while overlapping oblique, horizontal, or vertical strokes are used for adequate calculus removal.[38]
Current research on potentially more effective methods of subgingival calculus removal focuses on the use of near-ultraviolet (NUV) and near-infrared lasers, such as Er,Cr:YSGG lasers.[43][44] The use of lasers in periodontal therapy offers a unique clinical advantage over conventional hand instrumentation, as the thin and flexible fibers can deliver laser energy into periodontal pockets that are otherwise difficult to access.[44] Near-infrared lasers, such as the Er,CR:YSGG laser, have been proposed as an effective adjunct for calculus removal as the emission wavelength is highly absorbed by water, a large component of calculus deposits.[44] An optimal output power setting of 1.0-W with the near-infrared Er,Cr:YSGG laser has been shown to be effective for root scaling.[44] Near-ultraviolet (NUV) lasers have also shown promise as they allow the dental professional to remove calculus deposits quickly, without removing underlying healthy tooth structure, which often occurs during hand instrumentation.[43] Additionally, NUV lasers are effective at various irradiation angles for calculus removal.[43] Discrepancies in the efficiency of removal are due to the physical and optical properties of the calculus deposits, not to the angle of laser use.[43] Dental hygienists must receive additional theoretical and clinical training on the use of lasers, where legislation permits.[45]
See also
| Wikimedia Commons has media related to Dental calculus. |
References
- calx. Charlton T. Lewis and Charles Short. A Latin Dictionary on Perseus Project.
- χάλιξ. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- Harper, Douglas. "calculus". Online Etymology Dictionary. Harper, Douglas. "chalk". Online Etymology Dictionary.
- Harper, Douglas. "tartar". Online Etymology Dictionary.
- Jin Y, Yip HK (2002). "Supragingival calculus: formation and control" (PDF). Critical Reviews in Oral Biology and Medicine. 13 (5): 426–41. doi:10.1177/154411130201300506. PMID 12393761.
- Socransky SS, Haffajee AD (2002). "Dental biofilms: difficult therapeutic targets". Periodontology 2000. 28 (1): 12–55. doi:10.1034/j.1600-0757.2002.280102.x. PMID 12013340.
- Socransky SS, Haffajee AD (2005). "Periodontal microbial ecology". Periodontology 2000. 38 (1): 135–87. doi:10.1111/j.1600-0757.2005.00107.x. PMID 15853940.
- Warinner C, Speller C, Collins MJ (January 2015). "A new era in palaeomicrobiology: prospects for ancient dental calculus as a long-term record of the human oral microbiome". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 370 (1660): 20130376. doi:10.1098/rstb.2013.0376. PMC 4275884. PMID 25487328.
- Warinner C, Rodrigues JF, Vyas R, Trachsel C, Shved N, Grossmann J, et al. (April 2014). "Pathogens and host immunity in the ancient human oral cavity". Nature Genetics. 46 (4): 336–44. doi:10.1038/ng.2906. PMC 3969750. PMID 24562188.
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG (October 2010). "The human oral microbiome". Journal of Bacteriology. 192 (19): 5002–17. doi:10.1128/JB.00542-10. PMC 2944498. PMID 20656903.
- Warinner C, Hendy J, Speller C, Cappellini E, Fischer R, Trachsel C, et al. (November 2014). "Direct evidence of milk consumption from ancient human dental calculus". Scientific Reports. 4: 7104. Bibcode:2014NatSR...4E7104W. doi:10.1038/srep07104. PMC 4245811. PMID 25429530.
- Hardy K, Blakeney T, Copeland L, Kirkham J, Wrangham R, Collins M (2009). "Starch granules, dental calculus and new perspectives on ancient diet". Journal of Archaeological Science. 36 (2): 248–255. doi:10.1016/j.jas.2008.09.015.
- Blatt SH, Redmond BG, Cassman V, Sciulli PW (2011). "Dirty teeth and ancient trade: evidence of cotton fibres in human dental calculus from Late Woodland, Ohio". International Journal of Osteoarchaeology. 21 (6): 669–678. doi:10.1002/oa.1173.
- Hardy K, Buckley S, Collins MJ, Estalrrich A, Brothwell D, Copeland L, et al. (August 2012). "Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus". Die Naturwissenschaften. 99 (8): 617–26. Bibcode:2012NW.....99..617H. doi:10.1007/s00114-012-0942-0. hdl:10261/79611. PMID 22806252.
- Jepsen S, Deschner J, Braun A, Schwarz F, Eberhard J (February 2011). "Calculus removal and the prevention of its formation". Periodontology 2000. 55 (1): 167–88. doi:10.1111/j.1600-0757.2010.00382.x. PMID 21134234.
- Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG, Goodson JM (April 2005). "Phototargeting oral black-pigmented bacteria". Antimicrobial Agents and Chemotherapy. 49 (4): 1391–6. doi:10.1128/aac.49.4.1391-1396.2005. PMC 1068628. PMID 15793117.
- Schroeder HE (1969). Formation and Inhibition of Dental Calculus. Hans Huber Publishers. ISBN 9783456002354.
- Clayton YM, Fox EC (May 1973). "Investigations into the mycology of dental calculus in town-dwellers, agricultural workers and grazing animals". Journal of Periodontology. 44 (5): 281–5. doi:10.1902/jop.1973.44.5.281. PMID 4572515.
- Nelson KE, Fleischmann RD, DeBoy RT, Paulsen IT, Fouts DE, Eisen JA, et al. (September 2003). "Complete genome sequence of the oral pathogenic Bacterium porphyromonas gingivalis strain W83". Journal of Bacteriology. 185 (18): 5591–601. doi:10.1128/jb.185.18.5591-5601.2003. PMC 193775. PMID 12949112.
- Lieverse AR (1999). "Diet and the aetiology of dental calculus". Int. J. Osteoarchaeol. 9 (4): 219–232. doi:10.1002/(SICI)1099-1212(199907/08)9:4<219::AID-OA475>3.0.CO;2-V.
- White DJ (1991). "Processes contributing to the formation of dental calculus". Biofouling. 4 (1–3): 209–218. doi:10.1080/08927019109378211.
- White DJ (October 1997). "Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits". European Journal of Oral Sciences. 105 (5 Pt 2): 508–22. doi:10.1111/j.1600-0722.1997.tb00238.x. PMID 9395117.
- Nakano K, Nemoto H, Nomura R, Inaba H, Yoshioka H, Taniguchi K, Amano A, Ooshima T (February 2009). "Detection of oral bacteria in cardiovascular specimens". Oral Microbiology and Immunology. 24 (1): 64–8. doi:10.1111/j.1399-302x.2008.00479.x. PMID 19121072.
- Yeo BK, Lim LP, Paquette DW, Williams RC (January 2005). "Periodontal disease -- the emergence of a risk for systemic conditions: pre-term low birth weight". Annals of the Academy of Medicine, Singapore. 34 (1): 111–6. PMID 15726229.
- "Parameter on systemic conditions affected by periodontal diseases. American Academy of Periodontology". Journal of Periodontology. 71 (5 Suppl): 880–3. May 2000. doi:10.1902/jop.2000.71.5-S.880. PMID 10875699.
- Addy M, Richards J, Williams G (August 1980). "Effects of a zinc citrate mouthwash on dental plaque and salivary bacteria". Journal of Clinical Periodontology. 7 (4): 309–15. doi:10.1111/j.1600-051x.1980.tb01973.x. PMID 7007451.
- Gorrel C (December 1998). "Periodontal disease and diet in domestic pets". The Journal of Nutrition. 128 (12 Suppl): 2712S–2714S. doi:10.1093/jn/128.12.2712S. PMID 9868248.
- Hilson S (2005). Teeth. Cambridge University Press. ISBN 9780521545495.
- Diekwisch TG (September 2001). "The developmental biology of cementum". The International Journal of Developmental Biology. 45 (5–6): 695–706. PMID 11669371.
- Metcalf JL, Ursell LK, Knight R (April 2014). "Ancient human oral plaque preserves a wealth of biological data". Nature Genetics. 46 (4): 321–3. doi:10.1038/ng.2930. PMID 24675519.
- Warinner C, Rodrigues JF, Vyas R, Trachsel C, Shved N, Grossmann J, et al. (April 2014). "Pathogens and host immunity in the ancient human oral cavity". Nature Genetics. 46 (4): 336–44. doi:10.1038/ng.2906. PMC 3969750. PMID 24562188.
- Weyrich LS, Duchene S, Soubrier J, Arriola L, Llamas B, Breen J, et al. (April 2017). "Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus" (PDF). Nature. 544 (7650): 357–361. Bibcode:2017Natur.544..357W. doi:10.1038/nature21674. hdl:10261/152016. PMID 28273061.
- Adler CJ, Dobney K, Weyrich LS, Kaidonis J, Walker AW, Haak W, et al. (April 2013). "Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions". Nature Genetics. 45 (4): 450–5, 455e1. doi:10.1038/ng.2536. PMC 3996550. PMID 23416520.
- Radini A, Tromp M, Beach A, Tong E, Speller C, McCormick M, et al. (January 2019). "Medieval women's early involvement in manuscript production suggested by lapis lazuli identification in dental calculus". Science Advances. 5 (1): eaau7126. doi:10.1126/sciadv.aau7126. PMC 6326749. PMID 30662947.
- A. Molokhia and G. S. Nixon, "Studies on the composition of human dental calculus. Determination of some major and trace elements by instrumental neutron activation analysis", Journal Journal of Radioanalytical and Nuclear Chemistry, Volume 83, Number 2, August, 1984, p. 273-281. (abstract)
- "What is a Registered Dental Hygienist?". CRDHA. Archived from the original on 20 December 2016. Retrieved 16 December 2016.
- "Places Registered Dental Hygienists Work". CRDHA. Archived from the original on 20 December 2016. Retrieved 16 December 2016.
- Newman MG, Takei HH, Klokkevold PR, Carranza FA (2011). Carranza's Clinical Periodontology (11th ed.). St. Louis, Missouri: Saunders Book Company. p. 473. ISBN 978-1-4377-0416-7.
- Darby I (September 2009). "Non-surgical management of periodontal disease". Australian Dental Journal. 54 Suppl 1: S86–95. doi:10.1111/j.1834-7819.2009.01146.x. PMID 19737271.
- Westfelt E (March 1996). "Rationale of mechanical plaque control". Journal of Clinical Periodontology. 23 (3 Pt 2): 263–7. doi:10.1111/j.1600-051X.1996.tb02086.x. PMID 8707987.
- "Dental Care FAQs". Canadian Dental Association. Retrieved 16 December 2016.
- Kamath DG, Umesh Nayak S (January 2014). "Detection, removal and prevention of calculus: Literature Review". The Saudi Dental Journal. 26 (1): 7–13. doi:10.1016/j.sdentj.2013.12.003. PMC 3923169. PMID 24526823.
- Schoenly JE, Seka WD, Rechmann P (July 2011). "Near-ultraviolet removal rates for subgingival dental calculus at different irradiation angles". Journal of Biomedical Optics. 16 (7): 071404–071404–7. Bibcode:2011JBO....16g1404S. doi:10.1117/1.3564907. PMID 21806250.
- Ting CC, Fukuda M, Watanabe T, Aoki T, Sanaoka A, Noguchi T (November 2007). "Effects of Er,Cr:YSGG laser irradiation on the root surface: morphologic analysis and efficiency of calculus removal". Journal of Periodontology. 78 (11): 2156–64. doi:10.1902/jop.2007.070160. PMID 17970683.
- "HPA Frequently Asked Questions". CRDHA. Archived from the original on 20 December 2016. Retrieved 16 December 2016.