Dehydroretinal
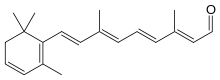
Dehydroretinal, more specifically, 3,4-dehydroretinal, is a derivative metabolite of retinal[1] belonging to the group of vitamin A2 as a retinaldehyde form, besides the endogenously present 3,4-dehydroretinol and 3,4-dehydroretinoic acid.[2][3]
 | |
 | |
| Names | |
|---|---|
| Other names
3,4–didehydroretinaldehyde | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.781 |
| EC Number |
|
| KEGG | |
| MeSH | Dehydroretinal |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C20H26O |
| Molar mass | 282.42 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The livers of some freshwater fishes and some fish found in India contain a higher ratio of dehydroretinal to retinal than do other species.[4][5]
References
- Gibney, Michael J.; Margetts, Barrie M.; Kearney, John M.; et al., eds. (2012), Public Health Nutrition, John Wiley & Sons, p. 210, ISBN 1118574222
- Törmä H, Vahlquist A (1985). "Biosynthesis of 3-dehydroretinol (vitamin A2) from all-trans-retinol (vitamin A1) in human epidermis". J. Invest. Dermatol. 85 (6): 498–500. doi:10.1111/1523-1747.ep12277290. PMID 4067325.
- Vahlquist A (1980). "The identification of dehydroretinol (vitamin A2) in human skin". Experientia. 36 (3): 317–318. doi:10.1007/bf01952299. PMID 7371787.
- MortonRA, Stubbs AL (1946). "Ling cod and other fish liver oils rich in vitamin A2". Biochem J. 40 (5–6): lix. PMID 20277273.
- Food and Agriculture Organization of the United Nations (1967), Requirements of Vitamin A, Thiamine, Riboflavin & Niacin: Report of a Joint Fao-Who Expert Group, United Nations, p. 26, ISBN 9251004536
See also
- Retinene
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.