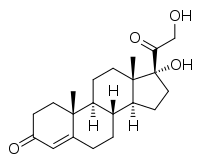
11-Deoxycortisol
11-Deoxycortisol, also known as cortodoxone (INN) or cortexolone, as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione,[1] is a glucocorticoid steroid hormone. It was first synthesized by Tadeusz Reichstein, and has also been referred to as Reichstein's Substance.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(8R,9S,10R,13S,14S,17R)-17-Hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one | |
| Other names
11-Deoxycortisol; 11-Deoxycortisone; Cortoxelone; 17α,21-Dihydroxypregn-4-ene-3,20-dione; 17α,21-Dihydroxyprogesterone; 11-Desoxycortisol; 11-Deoxyhydrocortisone; 11-Desoxyhydrocortisone; 17α-Hydroxy-11-deoxycorticosterone; Reichstein's Substance S; Compound S; Cortodoxone; Cortexolone, | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.279 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C21H30O4 |
| Molar mass | 346.467 g·mol−1 |
| Melting point | 215 °C (419 °F; 488 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
On April 5, 1952, biochemist Durey Peterson and microbiologist Herbert Murray at Upjohn published the first report of a breakthrough fermentation process for the microbial 11α-oxygenation of steroids (e.g. progesterone) in a single step by common molds of the order Mucorales.[2] 11α-oxygenation of Compound S produces 11α-hydrocortisone, which can be chemically oxidized to cortisone, or converted by further chemical steps to 11β-hydrocortisone (cortisol).
11-Deoxycortisol acts as a glucocorticoid, though is less potent than cortisol. It can be synthesized from 17α-hydroxyprogesterone. In 11β-hydroxylase deficiency, 11-deoxycortisol levels increase dramatically, causing hypertension (as opposed to 21-hydroxylase deficiency, in which patients have low blood pressure from a lack of mineralocorticoids).
11-Deoxycortisol can also be converted to androstenedione.[3] This could explain, at least in part, the marked increase in androstenedione levels in 11β-hydroxylase deficiency.[3]
See also
- 11-Deoxycorticosterone
- Cortexolone 17α-propionate
References
- R.A. Hill; H.L.J. Makin; D.N. Kirk; G.M. Murphy (23 May 1991). Dictionary of Steroids. CRC Press. pp. 338–. ISBN 978-0-412-27060-4.
- Peterson DH; Murray, HC (1952). "Microbiological oxygenation of steroids at carbon 11". J Am Chem Soc. 74 (7): 1871–2. doi:10.1021/ja01127a531.
- Auzéby A, Bogdan A, Touitou Y (January 1991). "Evidence for a new biologic pathway of androstenedione synthesis from 11-deoxycortisol". Steroids. 56 (1): 33–6. doi:10.1016/0039-128X(91)90112-9. PMID 2028480.