Stratum corneum
The stratum corneum (Latin for 'horny layer') is the outermost layer of the epidermis. There has been a long-standing belief in dermatology that the stratum corneum consisted of dead cells (corneocytes), devoid of biological activity and function. The stratum corneum is now understood to be live tissue that performs protective and adaptive physiological functions including mechanical shear, impact resistance, water flux and hydration regulation, microbial proliferation and invasion regulation, initiation of inflammation through cytokine activation and dendritic cell activity, and selective permeability to exclude toxins, irritants, and allergens.[2] This layer is composed of 15–20 layers of flattened cells with no nuclei and cell organelles. Their cytoplasm shows filamentous keratin. These corneocytes are embedded in a lipid matrix composed of ceramides, cholesterol, and fatty acids.[3] Their properties depend on the component ratio of the three major components.[4]
| Stratum corneum | |
|---|---|
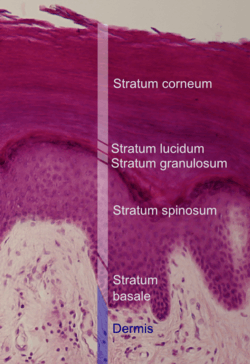 Histologic image of human epidermis in thick skin. | |
| Details | |
| Identifiers | |
| Latin | stratum corneum epidermidis |
| Anatomical terminology | |
The stratum corneum functions to form a barrier to protect underlying tissue from infection, dehydration, chemicals and mechanical stress. Desquamation, the process of cell shedding from the surface of the stratum corneum, balances proliferating keratinocytes that form in the stratum basale. These cells migrate through the epidermis towards the surface in a journey that takes approximately fourteen days.[5]
Function
During cornification, the process whereby living keratinocytes are transformed into non-living corneocytes, the cell membrane is replaced by a layer of ceramides which become covalently linked to an envelope of structural proteins (the cornified envelope).[5][6] This complex surrounds cells in the stratum corneum and contributes to the skin's barrier function. Corneodesmosomes (modified desmosomes) facilitate cellular adhesion by linking adjacent cells within this epidermal layer. These complexes are degraded by proteases, eventually permitting cells to be shed at the surface. Desquamation and formation of the cornified envelope are both required for the maintenance of skin homeostasis. A failure to correctly regulate these processes leads to the development of skin disorders.[5]
Cells of the stratum corneum contain a dense network of keratin, a protein that helps keep the skin hydrated by preventing water evaporation. These cells can also absorb water, further aiding in hydration. In addition, this layer is responsible for the "spring back" or stretchy properties of skin. A weak glutenous protein bond pulls the skin back to its natural shape.
The thickness of the stratum corneum varies throughout the body. In the palms of the hands and the soles of the feet (sometimes knees, elbows,[7] knuckles,) this layer is stabilized and built by the stratum lucidum (clear phase) which allows the cells to concentrate keratin and toughen them before they rise into a typically thicker, more cohesive SC. The mechanical stress of heavy structural strain causes this SL phase in these regions which require additional protection in order to grasp objects, resist abrasion or impact, and avoid injury. In general, the stratum corneum contains 15 to 20 layers of dead cells. The stratum corneum has a thickness between 10 and 40 μm.
In reptiles, the stratum corneum is permanent, and is replaced only during times of rapid growth, in a process called ecdysis or moulting. This is conferred by the presence of beta-keratin, which provides a much more rigid skin layer.
In the human forearm, about 1300 cells per cm2 per hour are shed. Stratum corneum protects the internal structures of the body from external injury and bacterial invasion.
Skin disease
An inability to correctly maintain the skin barrier function due to the dysregulation of epidermal components can lead to skin disorders. For example, a failure to modulate the activity of kallikreins via the disruption of the protease inhibitor LEKTI causes the debilitating disorder Netherton syndrome.[8]
See also
- Epidermis (skin)
- Stratum granulosum
- Stratum spinosum
- stratum corneum tryptic enzyme, old name for KLK5
References
- Sadowski T, Klose C, Gerl MJ, Wójcik-Maciejewicz A, Herzog R, Simons K, Reich A, Surma MA (2017). "Large-scale human skin lipidomics by quantitative, high-throughput shotgun mass spectrometry". Scientific Reports. 7: 43761. doi:10.1038/srep43761. PMC 5339821. PMID 28266621.
- Del Rosso, James Q.; Levin, Jacqueline (2011). "The Clinical Relevance of Maintaining the Functional Integrity of the Stratum Corneum in both Healthy and Disease-affected Skin". The Journal of Clinical and Aesthetic Dermatology. 4 (9): 22–42. ISSN 1941-2789. PMC 3175800. PMID 21938268.
- Mitra, Ashim K.; Kwatra, Deep; Vadlapudi, Aswani Dutt (2015). Drug Delivery. Burlington, MA: Jones & Bartlett Learning. pp. 285–286. ISBN 978-1-284-02568-2.
- Podewitz, Maren; Wang, Yin; Gkeka, Paraskevi; von Grafenstein, Susanne; Liedl, Klaus R.; Cournia, Zoe (11 October 2018). "Phase Diagram of a Stratum Corneum Lipid Mixture". The Journal of Physical Chemistry B. 122 (46): 10505–10521. doi:10.1021/acs.jpcb.8b07200. PMID 30351111.
- Ovaere P; Lippens S; Vandenabeele P; Declercq W. (2009). "The emerging roles of serine protease cascades in the epidermis". Trends in Biochemical Sciences. 34 (9): 453–463. doi:10.1016/j.tibs.2009.08.001. PMID 19726197.
- Haftek M; Callejon S; Sandjeu Y; Padois K; Falson F; Pirot F; Portes P; Demarne F; Jannin V. (2011). "Compartmentalization of the human stratum corneum by persistent tight junction-like structures". Exp Dermatol. 20 (8): 617–21. doi:10.1111/j.1600-0625.2011.01315.x. PMID 21672033.
- Dr. Raelene V. Shippee-Rice. "Gerioperative Nursing Care: Principles and Practices of Surgical Care". p. 322.
- Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, Elias P, Barrandon Y, Zambruno G, Sonnenberg A, Hovnanian A (Jan 2005). "Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity". Nat Genet. 37 (1): 56–65. doi:10.1038/ng1493. PMID 15619623.
External links
- MedEd at Loyola medicine/dermatology/melton/skinlsn/stcorn.htm
- Histology image: 08422loa – Histology Learning System at Boston University - "Integument: thick skin"