Copper peptide GHK-Cu
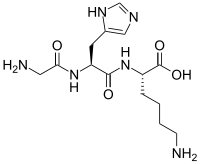
Copper peptide GHK-Cu is a naturally occurring copper complex of the tripeptide glycyl-L-histidyl-L-lysine. The tripeptide has strong affinity for copper(II) and was first isolated from human plasma. It can be found also in saliva and urine.
 Tripeptide | |
| Names | |
|---|---|
| IUPAC name
6-Amino-2-[[2-[(2-aminoacetyl)amino]-3-(1H-imidazol-5-yl)propanoyl]amino]hexanoic acid | |
| Other names
Glycyl-L-Histidyl-L-Lysine; Growth-modulating peptide; Kollaren; Liver cell growth factor; Liver growth factor Cu-GHK; Glycyl-histidyl-lysine, monocopper salt | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
PubChem CID |
|
SMILES
| |
| Properties | |
Chemical formula |
C14H24N6O4 C14H22CuN6O4 (Cu complex) |
| Molar mass | 340.38 g/mol |
Solubility in water |
130.98 g/L [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Overview
Several copper(II)-peptide complexes occur naturally.[2] In human plasma, the level of GHK-Cu is about 200 ng/ml at age 20. By the age of 60, the level drops to 80 ng/ml. In humans, GHK-Cu is proposed to promote wound healing, attraction of immune cells, antioxidant and anti-inflammatory effects, stimulation of collagen and glycosaminoglycan synthesis in skin fibroblasts and promotion of blood vessels growth. Recent studies revealed its ability to modulate expression of a large number of human genes, generally reversing gene expression to a healthier state. Synthetic GHK-Cu is used in cosmetics as a reparative and anti-aging ingredient.[3]
History
Loren Pickart isolated the copper peptide GHK-Cu from human plasma albumin in 1973.[4] It was noticed that liver tissue obtained from patients aged 60 to 80 years had an increased level of fibrinogen. However, when liver cells from old patients were incubated in the blood from the younger group, the older cells started functioning in nearly the same way as the younger liver tissue.[5][6] It turned out that this effect was due to a small peptide factor that behaved similarly to the synthetic peptide glycyl-L-histidyl-L-lysine (GHK). Pickart proposed that this activity in human plasma albumin was a tripeptide glycyl-L-histidyl-L-lysine and that it might function by chelating metal ions.[7]
In 1977, the growth modulating peptide was shown to be a glycyl-L-histidyl-L-lysine.[8] It is proposed that GHK-Cu modulates copper intake into cells.[9]
Wound healing
Biochemical studies
In the late 1980s, copper peptide GHK-Cu started attracting attention as a promising wound healing agent. At picomolar to nanomolar concentrations, GHK-Cu stimulated the synthesis of collagen in skin fibroblasts, increased accumulation of total proteins, glycosaminoglycans (in a biphasic curve)and DNA in the dermal wounds in rats. They also found out that the GHK sequence is present in collagen and suggested that the GHK peptide is released after tissue injury.[10][11] They proposed a class of emergency response molecules which are released from the extracellular matrix at the site of an injury.[12] GHK-Cu also increased synthesis of decorin – a small proteoglycan involved in the regulation of collagen synthesis, wound healing regulation and anti-tumor defense.[13]
It was also established that GHK-Cu stimulates both the synthesis of metalloproteinases, the enzymes which break down dermal proteins, and their inhibitors (anti-proteases). The fact that GHK-Cu not only stimulates the production of dermal components, but also regulates their breakdown suggests that it should be used with caution.[14]
Wound healing in animals
A series of animal experiments established pronounced wound healing activity of GHK-Cu. In the dermal wounds of rabbits GHK-Cu facilitated wound healing, causing better wound contraction, faster development of granular tissue and improved angiogenesis. It also elevated the level of antioxidant enzymes.[15][16]
GHK-Cu has been found to induce a systemic enhancement of healing in rats, mice, and pigs; that is, the GHK-Cu peptide injected in one area of the body (such as the thigh muscles) improved healing at distant body areas (such as the ears). These treatments strongly increased healing parameters such as collagen production, angiogenesis, and wound closure in both wound chambers and full thickness wounds.[17] In one study, full‐thickness wounds of 6 millimeters in diameter were created in an ischemic skin flap on the backs of rats, and for 13 days the wound sites were then treated daily with topical GHK or topical hydroxypropyl methylcellulose vehicle, or given no treatment. At the end of the study, the wound size had decreased by 64.5% in the GHK group; by 45.6% in the vehicle-treated group; and by 28.2% in the control group.[18] The difference between the GHK group's wounds and those of the control group was significant, and was accompanied by significantly lower levels of tumor necrosis factor alpha and elastin-degrading matrix metalloproteinases.[18]
Biotinylated GHK-Cu was incorporated into a collagen membrane, which was used as a wound dressing. This GHK-Cu enriched material stimulated wound contraction and cell proliferation, as well as increased expression of antioxidant enzymes. The same material was tested for wound healing in diabetic rats. GHK-Cu treatment resulted in faster wound contraction and epithelization, higher level of glutathione and ascorbic acid, increased synthesis of collagen, and activation of fibroblasts and mast cells.[19] Ischemic open wounds in rats treated with GHK-copper healed faster and had decreased concentration of metalloproteinases 2 and 9 as well as of tumor necrosis factor-beta (a major inflammatory cytokine) compared with vehicle alone or with untreated wounds.[18]
Human trials
A 2% GHK gel showed promising results in treatment of 120 diabetic patients, increasing the percentage of ulcer closure from 60.8% to 98.5%, and decreasing the percentage of infection from 34% to 7%. The rate of healing was three times greater with GHK.[20] However, a 0.4% GHK-Cu cream failed to reach therapeutic goal in treatment of venous ulcers.[21]
Current research
Anti-inflammatory activity
GHK peptide has anti-inflammatory properties but the mechanism remains unclear. GHK and its copper complexes decreased TNF-alpha-dependent IL-6 secretion in normal human dermal fibroblasts. Because of the anti-inflammatory properties, copper-peptides could replace corticosteroids or non-steroidal anti-inflammatory drugs in treatment of inflammatory skin conditions. They also can reduce UV-induced Erythema.[22]
DNA repair
Radioactive anti-cancer treatment slows cell replication by breaking DNA strands. A recent study showed GHK-Cu's ability to restore function of irradiated fibroblasts to that of intact cells. The researchers used cultured human fibroblasts obtained from cervical skin that was either intact or exposed to radioactive treatment (5000 rad). At a very low (1 nanomolar) concentration, GHK-Cu stimulated irradiated fibroblasts growth and increased their production of growth factors bFGF and VGF to the point where it became even higher than that of both the irradiated and intact control cells.[23]
Nerve regeneration
GHK promotes nerve regeneration. Axon regeneration was studied using collagen tubes with incorporated peptides. GHK increased migration of hematogenous cells into collagen tube, production of nerve growth factors, expression of integrins and the rate of regeneration of myelinated nerve fibers. In addition, GHK also increased axon count and proliferation of Schwann cells compared to the control.[24]
Effect on stem cells
GHK-Cu stimulates proliferation of keratinocytes and increased expression of integrins and p63 protein in the epidermal stem cells. Since p63 is considered to be an important marker of stem cell and anti-senescence protein, the authors concluded that GHK-copper is able to recover epidermal stem cells and increase their ability to repair tissue.[25] Similar activity was observed for copper-free GHK.[26]
Anti-cancer effect
GHK-Cu reverses the expression of certain genes involved in metastatic spreading of colon cancer. GHK-Cu was effective at a very low concentration - 1mkM.[27]
Genomic studies
GHK may directly modulate gene expression, which may explain the diversity of its biological actions. A repository of transcriptional responses to compounds, the Connectivity Map (cMap),[28] and MANTRA software to explore networks of compounds producing similar transcriptional responses. GHK, as one of the compounds studied, increased mRNA production in 268 genes while suppressing 167.[29] GHK was found to reverse the gene-expression signature of emphysematous destruction found in lung tissue obtained from smokers with COPD (Chronic Obstructive Pulmonary Disease). The gene expression signature associated with emphysema severity included 127 genes, involved in inflammation and repair. Using the Connectivity Map, researchers established that the peptide GHK downregulated genes involved in lung destruction and inflammation, while upregulating genes involved in tissue repair. Addition of 10 nanomolar GHK to lung fibroblasts from emphysema lungs restored their ability to remodel collagen and assemble it into properly organized fibrils.[30]
Cosmetic use
Facial studies
Copper peptide GHK-Cu is widely used in anti-aging cosmetics (INCI name: Copper tripeptide-1).[31] Several controlled facial studies confirmed anti-aging, firming and anti-wrinkle activity of copper peptide GHK-Cu.
Facial cream containing GHK-Cu and melatonin increased collagen in photoaged skin of 20 female volunteers, performing better than vitamin C and retinoic acid.[32] The study was not controlled for cream vehicle application alone without active ingredients.
A 12-week facial study on 67 women indicated that GHK-Cu cream applied twice daily improved aged skin appearance, increased thickness, reduced wrinkles and strongly stimulated dermal keratinocyte proliferation as determined by histological analysis of biopsies. The same study found copper peptide GHK-Cu to be non-toxic and non-irritating.[33]
Hair growth
Copper peptide GHK-Cu and its analogues were found to stimulate hair growth. In some circumstances, the efficiency of synthetic analog of GHK-Cu was similar to that of 5% minoxidil.[34] A commercial product GraftCyte was clinically proven to improve hair transplantation outcome.[35] Shown to promote collagen production, using copper peptides topically on the scalp will help strengthen already existing hair, while stimulating growth in areas that are lacking thickness.
Biological chemistry
Copper binding
Replacement of histidine with other amino acids showed that the glycine residue plays major role in copper binding, whereas lysine can interact with copper only at alkaline pH. At physiological pH, lysine is able to interact with a cellular receptor. The ability of GHK to interact both with copper and with a cellular receptor may allows it to transfer copper into and from cells. The small size of GHK permits speedy traveling in extracellular space and its easy access to cellular receptors.[36]
The molecular structure of the GHK copper complex (GHK-Cu) has been determined X-ray crystallography, EPR spectroscopy, X-ray absorption spectroscopy, NMR spectroscopy, as well as other methods such as titration. In the GHK-Cu complex, the Cu (II) ion is coordinated by the nitrogen from the imidazole side chain of the histidine, another nitrogen from the alpha-amino group of glycine and the deprotonated amide nitrogen of the glycine–histidine peptide bond. Since such a structure couldn't explain a high stability constant of the GHK-Cu complex (log 10 =16.44 vs. 8.68 of the GH copper complex, which is similar to the GHK-Cu structure), it was proposed that another amino group participates in the complex formation. Cu(II) is also coordinated by the oxygen from the carboxyl group of the lysine from the neighboring complex. Another carboxyl group of lysine from a neighboring complex provides the apical oxygen, resulting in the square-planar pyramid configuration.[37] Many researchers proposed that at the physiological pH, GHK-Cu complexes can form binary and ternary structures which may involve amino acid histidine and/or the copper binding region of the albumin molecule. Lau and Sarkar found also that GHK can easily obtain copper 2+ bound to other molecules such as the high affinity copper transport site on plasma albumin (albumin binding constant log 10 =16.2 vs. GHK binding constant 16 log 10 =16.44). It has been established that copper (II) redox activity is silenced when copper ions are complexed with the GHK tripeptide, which allows the delivery of non-toxic copper into the cell.[38]
Biological significance
Copper is vital for all eukaryotic organisms from microbes to humans. A dozen enzymes (cuproenzymes) use changes in copper oxidation state to catalyze important biochemical reactions including cellular respiration (cytochrome c oxidase), antioxidant defense (ceruloplasmin, superoxide dismutase (SOD), detoxification (metallothioneins), blood clotting (blood clotting factors V and VIII), melanin production (tyrosinase) and the connective tissue formation (lysyl peroxidase). Copper is required for iron metabolism, oxygenation, neurotransmission, embryonic development and many other essential biological processes. Another function of copper is signaling – for example, stem cells require a certain level of copper in the media to start their differentiation into cells needed for repair. Thus, GHK-Cu's ability to bind copper and to modulate its tissue level is a key factor determining its biological activity.[39]
References
- "Archived copy" (PDF). Archived from the original (PDF) on 2012-03-24. Retrieved 2011-05-15.CS1 maint: archived copy as title (link)
- http://www.copper-peptides.com/Science.html
- Pickart, L (2008). "The human tri-peptide GHK and tissue remodeling". Journal of Biomaterials Science, Polymer Edition. 19 (8): 969–988. doi:10.1163/156856208784909435. PMID 18644225.
- Pickart, L; Thaler, MM (1973). "Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver". Nature New Biology. 243 (124): 85–87. PMID 4349963.
- Pilgeram, L; Pickart, L (1968). "Control of fibrinogen biosynthesis; the role of free fatty acids". Journal of Atherosclerosis Research. 8 (1): 155–166. doi:10.1016/s0368-1319(68)80089-4. PMID 5642099.
- Pilgeram, L (2010). "Control of fibrinogen biosynthesis; role of FFA/Albumin Ratio". Cardiovascular Engineering. 10 (2): 78–83. doi:10.1007/s10558-010-9092-1. PMC 2885297. PMID 20383582.
- Pickart, L (1973), A tripeptide in human plasma that increases the survival of hepatocytes and the growth of hepatoma cells, Ph.D. Thesis in Biochemistry: University of California, San Francisco
- Schlesinger, DH; Pickart, L; Thaler, MM (1977). "Growth-modulating serum tripeptide is glycyl-histidyl-lysine". Cellular and Molecular Life Sciences. 33 (3): 324–325. doi:10.1007/BF02002806. PMID 858356.
- Pickart, L; Freedman, JH; Loker, WJ; et al. (1980). "Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells". Nature. 288 (5792): 715–717. Bibcode:1980Natur.288..715P. doi:10.1038/288715a0. PMID 7453802.
- Maquart, FX; Pickart, L; Laurent, M; Gillery, P; Monboisse, JC; Borel, JP (1988). "Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+". FEBS Letters. 238 (2): 343–6. doi:10.1016/0014-5793(88)80509-x. PMID 3169264.
- Wegrowski, Y.; Maquart, F.X.; Borel, J.P. (1992). "Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex Glycyl-L-histidyl-L-lysine-Cu2+". Life Sciences. 51 (13): 1049–1056. doi:10.1016/0024-3205(92)90504-i. PMID 1522753.
- Maquart, FX; Bellon, G; Pasco, S; Monboisse, JC (2005). "Matrikines in the regulation of extracellular matrix degradation". Biochimie. 87 (3–4): 353–60. doi:10.1016/j.biochi.2004.10.006. PMID 15781322.
- Siméon, A; Wegrowski, Y; Bontemps, Y; Maquart, FX (2000). "Expression of glycosaminoglycans and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu(2+)". The Journal of Investigative Dermatology. 115 (6): 962–8. doi:10.1046/j.1523-1747.2000.00166.x. PMID 11121126.
- Siméon, Alain; Emonard, Hervé; Hornebeck, William; Maquart, François-Xavier (2000). "The tripeptide-copper complex glycyl-L-histidyl-L- lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures". Life Sciences. 67 (18): 2257–2265. doi:10.1016/s0024-3205(00)00803-1. PMID 11045606.
- Gul, NY; Topal, A; Cangul, IT; Yanik, K (2008). "The effects of topical tripeptide copper complex and helium-neon laser on wound healing in rabbits". Veterinary Dermatology. 19 (1): 7–14. doi:10.1111/j.1365-3164.2007.00647.x. PMID 18177285.
- Cangul, IT; Gul, NY; Topal, A; Yilmaz, R (2006). "Evaluation of the effects of topical tripeptide-copper complex and zinc oxide on open-wound healing in rabbits". Veterinary Dermatology. 17 (6): 417–23. doi:10.1111/j.1365-3164.2006.00551.x. PMID 17083573.
- Pickart L. Compositions for accelerating wound healing in mammals containing cupric salt or complexes with amino acid or peptide. US Patent 5,164,367, 1992.
- Canapp SO Jr, Farese JP, Schultz GS, Gowda S, Ishak AM, Swaim SF, Vangilder J, Lee-Ambrose L, Martin FG (Nov–Dec 2003). "The effect of topical tripeptide-copper complex on healing of ischemic open wounds". Veterinary Surgery. 32 (6): 515–23. doi:10.1111/j.1532-950x.2003.00515.x. PMID 14648529.CS1 maint: multiple names: authors list (link) CS1 maint: date format (link)
- Kartha, R; Jayakumar, R (2007). "A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices". Life Sciences. 80 (4): 275–84. doi:10.1016/j.lfs.2006.09.018. PMID 17049946.
- Mulder DPM1, Gerit D.; Patt PhD2, Leonard M.; Sanders DPM, Lee; et al. (1994). "Enhanced healing of ulcers in patients with diabetes by topical treatment of glycyl-l-histidyl-l-lysine". Wound Repair and Regeneration. 2 (4): 259–269. doi:10.1046/j.1524-475X.1994.20406.x. PMID 17147644.
- Bishop, JB; Phillips, LG; Mustoe, TA; VanderZee, AJ; Wiersema, L; Roach, DE; Heggers, JP; Hill Jr, DP; Taylor, EL; Robson, MC (Aug 1992). "A prospective randomized evaluator-blinded trial of two potential wound healing agents for the treatment of venous stasis ulcers". Journal of Vascular Surgery. 16 (2): 251–257. doi:10.1016/0741-5214(92)90115-o. PMID 1495150.
- Gruchlik, A.; Jurzak, M.; Chodurek, E.; Dzierzewicz, Z. (2012). "Effect of Gly-Gly-His, Gly-His-Lys and their copper complexes on TNF-alpha-dependent IL-6 secretion in normal human dermal fibroblasts". Acta Poloniae Pharmaceutica. 69 (6): 1303–6. PMID 23285694.
- Pollard, JD; Quan, S; Kang, T; Koch, RJ (2005). "Effects of copper tripeptide on the growth and expression of growth factors by normal and irradiated fibroblasts". Archives of Facial Plastic Surgery. 7 (1): 27–31. doi:10.1001/archfaci.7.1.27. PMID 15655171.
- Ahmed, M.R.; Basha, S.H.; Gopinath, D.; Muthusamy, R.; Jayakumar, R. (2005). "Initial upregulation of growth factors and inflammatory mediators during nerve regeneration in the presence of cell adhesive peptide-incorporated collagen tubes". Journal of the Peripheral Nervous System. 10 (1): 17–30. doi:10.1111/j.1085-9489.2005.10105.x. PMID 15703015.
- Kang, YA; Choi, HR; Na, JI; Huh, CH; Kim, MJ; Youn, SW; Kim, KH; Park, KC (Apr 2009). "Copper-GHK increases integrin expression and p63 positivity by keratinocytes". Archives of Dermatological Research. 301 (4): 301–6. doi:10.1007/s00403-009-0942-x. PMID 19319546.
- Choi, H.R.; Kang, Y.A.; Ryoo, S.J.; Shin, J.W.; Na, J.I.; Huh, C.H.; Park, K.C. (Nov 2012). "Stem cell recovering effect of copper-free GHK in skin". Journal of Peptide Science. 18 (11): 685–90. doi:10.1002/psc.2455. PMID 23019153.
- Hong, Y; Downey, T; Eu, KW; Koh, PK; Cheah, PY (2010). "A 'metastasis-prone' signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics". Clinical & Experimental Metastasis. 27 (2): 83–90. doi:10.1007/s10585-010-9305-4. PMID 20143136.
- Lamb, J (2007). "The Connectivity Map: a new tool for biomedical research". Nature Reviews Cancer. 7 (1): 54–60. doi:10.1038/nrc2044. PMID 17186018.
- Iorio, F.; Bosotti, R.; Scacheri, E.; et al. (2010). "Discovery of drug mode of action and drug repositioning from transcriptional responses". Proceedings of the National Academy of Sciences. 107 (33): 14621–14626. Bibcode:2010PNAS..10714621I. doi:10.1073/pnas.1000138107. PMC 2930479. PMID 20679242.
- Campbell, J.D.; McDonough, J.E.; Zeskind, J.E.; Hackett, T.L.; Pechkovsky, D.V.; Brandsma, C.A.; Suzuki, M.; Gosselink, J.V.; Liu, G.; Alekseyev, Y.O.; Xiao, J.; Zhang, X.; Hayashi, S.; Cooper, J.D.; Timens, W.; Postma, D.S.; Knight, D.A.; Marc, L.E.; James, H.C.; Avrum, S. (2012). "A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK". Genome Medicine. 4 (8): 67. doi:10.1186/gm367. PMC 4064320. PMID 22937864.
- Gorouhi, F.; Maibach, H.I. (2009). "Role of topical peptides in preventing and treating aged skin". International Journal of Cosmetic Science. 31 (5): 327–345. doi:10.1111/j.1468-2494.2009.00490.x. PMID 19570099.
- Abdulghani, AA; Sherr, S; Shirin, S; Solodkina, G; Tapia, EM; Gottlieb, AB (1998). "Effects of topical creams containing vitamin C, a copper-binding peptide cream and melatonin compared with tretinoin on the ultrastructure of normal skin - A pilot clinical, histologic, and ultrastructural study". Disease Management and Clinical Outcomes. 1: 136–141. doi:10.1016/S1088-3371(98)00011-4.
- Finkley MB, Appa Y, Bhandarkar S. Copper Peptide and Skin. Cosmeceuticals and Active Cosmetic, 2nd Edition, P. Eisner and H.I. Maibach (Eds.) Marcel Dekker, New York. 2005:549-563
- Uno, Hideo; Kurata, Sotaro (1993). "Chemical Agents and Peptides Affect Hair Growth". Journal of Investigative Dermatology. 101 (1 Suppl): 143S–147S. doi:10.1111/1523-1747.ep12363275. PMID 8326148.
- Perez-Meza, D; Leavitt, M; Trachy, R (1988). "Clinical evaluation of GraftCyte moist dressings on hair graft viability and quality of healing". International Journal of Cosmetic Surgery. 6: 80–84.
- Conato, Chiara; Gavioli, Riccardo; Guerrini, Remo; Kozłowski, Henryk; Młynarz, Piotr; Pasti, Claudia; Pulidori, Fernando; Remelli, Maurizio (2001). "Copper complexes of glycyl-histidyl-lysine and two of its synthetic analogues: Chemical behaviour and biological activity". Biochimica et Biophysica Acta (BBA) - General Subjects. 1526 (2): 199–210. doi:10.1016/s0304-4165(01)00127-1. PMID 11325542.
- Hureau, C.; Eury, H.; Guillot, R.; Bijani, C.; Sayen, S.; Solari, P.L.; Guillon, E.; Faller, P.; Dorlet, P (2011). "X-ray and solution structures of Cu(II) GHK and Cu(II) DAHK complexes: influence on their redox properties". Chemistry: A European Journal. 17 (36): 10151–60. doi:10.1002/chem.201100751. PMID 21780203.
- Lau, S.J.; Sarkar, B. (1981). "The interaction of copper(II) and glycyl-L-histidyl-L-lysine, a growth-modulating tripeptide from plasma". Biochemical Journal. 199 (3): 649–56. doi:10.1042/bj1990649. PMC 1163421. PMID 7340824.
- Pickart L. The human tripeptide GHK (Glycyl-L-histidyl-L-Lysine), the copper switch and the treatment of the degenerative conditions of aging. In Anti-Aging Therapeutics Volume XI, 301-3012. Ed. By Klatz R. and Goldman R. Chicago, IL, USA: American Academy of Medicine, 2009