Congenital diaphragmatic hernia
Congenital diaphragmatic hernia (CDH) is a birth defect of the diaphragm. The most common type of CDH is a Bochdalek hernia; other types include Morgagni hernia, diaphragm eventration and central tendon defects of the diaphragm. Malformation of the diaphragm allows the abdominal organs to push into the chest cavity, hindering proper lung formation.
| Congenital diaphragmatic hernia | |
|---|---|
| Morgagni hernia seen on a chest radiograph. | |
| Specialty | Medical genetics, pediatrics |
CDH is a life-threatening pathology in infants and a major cause of death due to two complications: pulmonary hypoplasia and pulmonary hypertension.[1] Experts disagree on the relative importance of these two conditions, with some focusing on hypoplasia, others on hypertension.[2] Newborns with CDH often have severe respiratory distress which can be life-threatening unless treated appropriately.
Classification
Bochdalek hernia
The Bochdalek hernia, also known as a postero-lateral diaphragmatic hernia, is the most common manifestation of CDH, accounting for more than 95% of cases. In this instance the diaphragm abnormality is characterized by a hole in the postero-lateral corner of the diaphragm which allows passage of the abdominal viscera into the chest cavity. The majority of Bochdalek hernias (80–85%) occur on the left side of the diaphragm, a large proportion of the remaining cases occur on the right side. To date, it carries a high mortality and is an active area of clinical research.
Morgagni hernia
This rare anterior defect of the diaphragm is variably referred to as a Morgagni, retrosternal, or parasternal hernia. Accounting for approximately 2% of all CDH cases, it is characterized by herniation through the foramina of Morgagni which are located immediately adjacent and posterior to the xiphoid process of the sternum.[3]
Diaphragm eventration
The diagnosis of congenital diaphragmatic eventration is used when there is abnormal displacement (i.e. elevation) of part or all of an otherwise intact diaphragm into the chest cavity. This rare type of CDH occurs because in the region of eventration the diaphragm is thinner, allowing the abdominal viscera to protrude upwards.
Pathophysiology
It involves three major defects:
- A failure of the diaphragm to completely close during development
- Herniation of the abdominal contents into the chest
- Pulmonary hypoplasia
Diagnosis
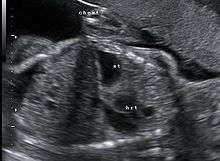
This condition can often be diagnosed before birth and fetal intervention can sometimes help, depending on the severity of the condition.[4] Infants born with diaphragmatic hernia experience respiratory failure due to both pulmonary hypertension and pulmonary hypoplasia. The first condition is a restriction of blood flow through the lungs thought to be caused by defects in the lung. Pulmonary hypoplasia or decreased lung volume is directly related to the abdominal organs presence in the chest cavity which causes the lungs to be severely undersized, especially on the side of the hernia.
Survival rates for infants with this condition vary, but have generally been increasing through advances in neonatal medicine. Work has been done to correlate survival rates to ultrasound measurements of the lung volume as compared to the baby's head circumference. This figure known as the lung to head ratio (LHR). Still, LHR remains an inconsistent measure of survival. Outcomes of CDH are largely dependent on the severity of the defect and the appropriate timing of treatment.
A small percentage of cases go unrecognized into adulthood.[5]
Treatment
The first step in management is orogastric tube placement and securing the airway (intubation). The baby will usually be immediately placed on a ventilator. Extracorporeal membrane oxygenation (ECMO) has been used as part of the treatment strategy at some hospitals.[6][7] ECMO acts as a baby heart-lung bypass (though it can be used for older children as well). A venous cannula is inserted into the jugular vein or the common femoral vein (ECMO is divided into two types; (arteriovenous AV and venovenous VV), allowing the blood to exit the body and begin its trek through the ECMO circuit, it is then scrubbed, oxygenated, and passes through a filter before being returned to the body via a second cannula into the baby’s own circulatory system where it makes its rounds before returning to the ECMO circuit to be oxygenated again. In essence, the ECMO circuit acts as the baby's lungs. Babies require extra blood volume and hefty doses of blood thinners in order to keep the circuit running without clot formation, which could be potentially fatal. Even though the baby is not using her lungs, an ocillating ventilator maybe still be used to keep some air in the lungs so that they do not fully collapse while not being used. During ECMO the pulmonary artery has a chance to rest, as it were, thus hopefully reducing the presence of pulmonary hypertension, one of the biggest complication of CDH cases. CDH repair can be done while the baby is on ECMO, although blood thinners increase the risk of bleeding complications. Usually surgeons prefer to perform CDH repairs off ECMO. Once the baby is taken off ECMO the carotid artery is sealed and can no longer be used. When repairing the hernia an incision is made in the abdomen. The hernia can sometimes be simply stitched closed but in more complicated cases a patch may be required. A synthetic patch can be used but will usually require replacement later as the child grows. A more natural patch can be created by slicing and folding over a section of abdominal muscle and securing it to the existing piece of diaphragm. Any organ displacement is corrected during surgery; the heart and lungs will usually move back into position on their own, once displaced organs such as bowel, liver, or stomach, are out of the way. The incision is then closed. Sometimes, the incision site will be left open to allow the body to adjust to newly moved organs and the pressure associated with that, and then closed later once swelling and drainage has decreased.[8]
Diaphragm eventration is typically repaired thoracoscopically, by a technique called plication of the diaphragm.[9] Plication basically involves a folding of the eventrated diaphragm which is then sutured in order to “take up the slack” of the excess diaphragm tissue.
Prognosis
Congenital diaphragmatic hernia has a mortality rate of 40–62%,[10] with outcomes being more favorable in the absence of other congenital abnormalities. Individual rates vary greatly dependent upon multiple factors: size of hernia, organs involved, additional birth defects, and/or genetic problems, amount of lung growth, age and size at birth, type of treatments, timing of treatments, complications (such as infections) and lack of lung function.
See also
- CHERUBS
- Diaphragmatic rupture
References
- Gaxiola A, Varon J, Valladolid G (April 2009). "Congenital diaphragmatic hernia: an overview of the etiology and current management". Acta Paediatrica. 98 (4): 621–7. doi:10.1111/j.1651-2227.2008.01212.x. PMID 19154527.
- Migliazza L, Bellan C, Alberti D, Auriemma A, Burgio G, Locatelli G, Colombo A (September 2007). "Retrospective study of 111 cases of congenital diaphragmatic hernia treated with early high-frequency oscillatory ventilation and presurgical stabilization". Journal of Pediatric Surgery. 42 (9): 1526–32. doi:10.1016/j.jpedsurg.2007.04.015. PMID 17848243.
- Arráez-Aybar LA, González-Gómez CC, Torres-García AJ (2009). "Morgagni-Larrey parasternal diaphragmatic hernia in the adult". Rev Esp Enferm Dig. 101 (5): 357–66. doi:10.4321/S1130-01082009000500009. PMID 19527083.
- "Deadly hernia corrected in womb – Surgeons have developed an operation to repair a potentially fatal abnormality in babies before they are born". BBC news. 2004-07-26. Retrieved 2006-07-14. – report of new operation, pioneered at London's King's College Hospital which reduced death rates in the most at risk by 50%
- Swain F, Klaus A, Achem S, Hinder R (2001). "Congenital Diaphragmatic Hernia in Adults". Surgical Innovation. 8 (4): 246–255. doi:10.1177/155335060100800404.
- Tiruvoipati R, Vinogradova Y, Faulkner G, Sosnowski AW, Firmin RK, Peek GJ (2007). "Predictors of outcome in patients with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation". J. Pediatr. Surg. 42 (8): 1345–50. doi:10.1016/j.jpedsurg.2007.03.031. PMID 17706494.
- Logan JW, Rice HE, Goldberg RN, Cotten CM (2007). "Congenital diaphragmatic hernia: a systematic review and summary of best-evidence practice strategies". Journal of Perinatology. 27 (9): 535–49. doi:10.1038/sj.jp.7211794. PMID 17637787.
- personal experience and talking with doctors, nurses, and surgeons at Primary Children's Medical Center in Salt Lake City, UT
- Becmeur F, Talon I, Schaarschmidt K, et al. (2005). "Thoracoscopic diaphragmatic eventration repair in children: about 10 cases". J. Pediatr. Surg. 40 (11): 1712–5. doi:10.1016/j.jpedsurg.2005.07.008. PMID 16291157.
- Pediatric Congenital Diaphragmatic Hernia at eMedicine
External links
| Classification | |
|---|---|
| External resources |
|
| Wikimedia Commons has media related to Congenital diaphragmatic hernia. |