Common Technical Document
The Common Technical Document (CTD) is a set of specifications for an application dossier for the registration of Medicines and designed to be used across Europe, Japan and the United States. It is an internationally agreed format for the preparation of applications regarding new drugs intended to be submitted to regional regulatory authorities in participating countries. It was developed by the European Medicines Agency (EMA, Europe), the Food and Drug Administration (FDA, US) and the Ministry of Health, Labour and Welfare (Japan). The CTD is maintained by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).[1]
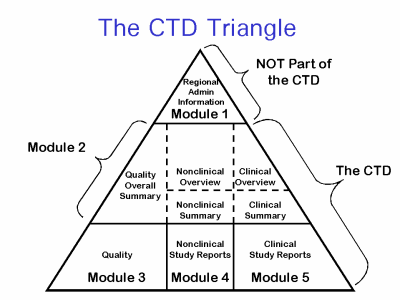
The Common Technical Document is divided into five modules:
- Administrative and prescribing information
- Overview and summary of modules 3 to 5
- Quality (pharmaceutical documentation)
- Preclinical (Pharmacology/Toxicology)
- Clinical – efficacy and safety (Clinical Trials)
Detailed subheadings for each Module are specified for all jurisdictions. The contents of Module 1 and certain subheadings of other Modules will differ, based on national requirements.
After the United States, European Union and Japan, the CTD has been adopted by several other countries including Canada and Switzerland.
The Paper CTD is destined to be replaced by its electronic counterpart, the eCTD.
See also
References
- https://www.fda.gov/cber/gdlns/m4ctd.pdf "Guidance for Industry, ICH M4: Organization of the CTD" U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) August 2001