Chlamydia pneumoniae
Chlamydia pneumoniae is a species of Chlamydia, an obligate intracellular bacterium[2] that infects humans and is a major cause of pneumonia. It was known as the Taiwan acute respiratory agent (TWAR) from the names of the two original isolates – Taiwan (TW-183) and an acute respiratory isolate designated AR-39.[3] Briefly, it was known as Chlamydophila pneumoniae, and that name is used as an alternate in some sources.[4] In some cases, to avoid confusion, both names are given.[5]
| Chlamydia pneumoniae | |
|---|---|
| Scientific classification | |
| Kingdom: | Bacteria |
| Phylum: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | C. pneumoniae[1] |
C. pneumoniae has a complex life cycle and must infect another cell to reproduce; thus, it is classified as an obligate intracellular pathogen. The full genome sequence for C. pneumoniae was published in 1999.[6] It also infects and causes disease in koalas, emerald tree boas (Corallus caninus), iguanas, chameleons, frogs, and turtles.
The first known case of infection with C. pneumoniae was a case of conjunctivitis in Taiwan in 1950. There are no known cases of C. pneumoniae in human history before 1950. This atypical bacterium commonly causes pharyngitis, bronchitis, coronary artery disease and atypical pneumonia in addition to several other possible diseases.[7][8]
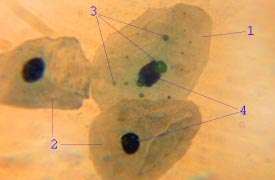
Life cycle and method of infection
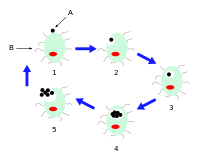
Chlamydia pneumoniae is a small gram negative bacterium (0.2 to 1 μm) that undergoes several transformations during its life cycle. It exists as an elementary body (EB) between hosts. The EB is not biologically active, but is resistant to environmental stresses and can survive outside a host for a limited time. The EB travels from an infected person to the lungs of an uninfected person in small droplets and is responsible for infection. Once in the lungs, the EB is taken up by cells in a pouch called an endosome by a process called phagocytosis. However, the EB is not destroyed by fusion with lysosomes, as is typical for phagocytosed material. Instead, it transforms into a reticulate body (RB) and begins to replicate within the endosome. The reticulate bodies must use some of the host's cellular metabolism to complete its replication. The reticulate bodies then convert back to elementary bodies and are released back into the lung, often after causing the death of the host cell. The EBs are thereafter able to infect new cells, either in the same organism or in a new host. Thus, the lifecycle of C. pneumoniae is divided between the elementary body, which is able to infect new hosts but cannot replicate, and the reticulate body, which replicates but is not able to cause new infection.
Diseases
C. pneumoniae is a common cause of pneumonia around the world; it is typically acquired by otherwise-healthy people and is a form of community-acquired pneumonia. Its treatment and diagnosis are different from historically recognized causes, such as Streptococcus pneumoniae.[9] Because it does not gram stain well, and because C. pneumoniae bacteria is very different from the many other bacteria causing pneumonia (in the earlier days, it was even thought to be a virus), the pneumonia caused by C. pneumoniae is categorized as an "atypical pneumonia".
One meta-analysis of serological data comparing prior C. pneumoniae infection in patients with and without lung cancer found results suggesting prior infection was associated with an increased risk of developing lung cancer.[10][11][12]
In research into the association between C. pneumoniae infection and atherosclerosis and coronary artery disease, serological testing, direct pathologic analysis of plaques, and in vitro testing suggest infection with C. pneumoniae is a significant risk factor for development of atherosclerotic plaques and Atherosclerosis.[13] C. pneumoniae infection increases adherence of macrophages to endothelial cells in vitro and aortas ex vivo.[14] However, most current research and data are insufficient and do not define how often C. pneumoniae is found in atherosclerotic or normal vascular tissue.[15]
C. pneumoniae has also been found in the cerebrospinal fluid of patients diagnosed with multiple sclerosis.[16]
C. pneumoniae infection was first associated with wheezing, asthmatic bronchitis, and adult-onset asthma in 1991.[17] Subsequent studies of bronchoalveolar lavage fluid from pediatric patients with asthma and also other severe chronic respiratory illnesses have demonstrated that over 50 percent had evidence of C. pneumoniae by direct organism identification.[18][19] C. pneumoniae infection triggers acute wheezing, if it becomes chronic then it is diagnosed as asthma.[20] These observations suggest that acute C. pneumoniae infection is capable of causing protean manifestations of chronic respiratory illness which lead to asthma.
C. pneumoniae infection is also associated with schizophrenia. Many other pathogens have been associated with schizophrenia.[21]
Macrolide antibiotic treatment can improve asthma in a subgroup of patients that remains to be clearly defined. Macrolide benefits were first suggested in two observational trials[22][23] and two randomized controlled trials[24][25] of azithromycin treatment for asthma. One of these RCTs[25] and another macrolide trial[26] suggest that the treatment effect may be greatest in patients with severe, refractory asthma. These clinical results correlate with epidemiological evidence that C. pneumoniae is positively associated with asthma severity[27] and laboratory evidence that C. pneumoniae infection creates steroid-resistance.[28] A recent meta analysis of 12 RCTs of macrolides for the long term management of asthma found significant effects on asthma symptoms, quality of life, bronchial hyper reactivity and peak flow but not FEV1.[29] Evidence from macrolide RCTs of patients with uncontrolled severe and refractory asthma will be critical in defining the role of macrolides in asthma.
Vaccine research
There is currently no vaccine to protect against Chlamydia pneumoniae. Identification of immunogenic antigens is critical for the construction of an efficacious subunit vaccine against C. pneumoniae infections. Additionally, there is a general shortage worldwide of facilities which can identify/diagnose Chlamydia pneumoniae.
References
- Everett KD, Bush RM, Andersen AA (April 1999). "Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms". International Journal of Systematic Bacteriology. 49 (2): 415–40. doi:10.1099/00207713-49-2-415. PMID 10319462.
- Chlamydia+pneumoniae at the US National Library of Medicine Medical Subject Headings (MeSH)
- Mayer G (24 June 2010). "Bacteriology - Chapter Twenty: Chlamydia and Chlamydophila". Bacteriology Section of Microbiology and Immunology On-line. University of South Carolina School of Medicine. Archived from the original on 2014-11-11.
- "Chlamydia pneumoniae". Taxonomy Browser. National Center for Biotechnology Information (NCBI), U.S. National Library of Medicine. Retrieved 2009-01-27.
- Appelt DM, Roupas MR, Way DS, Bell MG, Albert EV, Hammond CJ, Balin BJ (2008). "Inhibition of apoptosis in neuronal cells infected with Chlamydophila (Chlamydia) pneumoniae". BMC Neuroscience. 9: 13. doi:10.1186/1471-2202-9-13. PMC 2266938. PMID 18218130.
- Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman RW, Olinger L, Grimwood J, Davis RW, Stephens RS (April 1999). "Comparative genomes of Chlamydia pneumoniae and C. trachomatis". Nature Genetics. 21 (4): 385–9. doi:10.1038/7716. PMID 10192388.
- Lang BR (September 15, 1991). "Chlamydia pneumonia as a differential diagnosis? Follow-up to a case report on progressive pneumonitis in an adolescent". Patient Care.
- Little L (September 19, 1991). "Elusive pneumonia strain frustrates many clinicians". Medical Tribune: 6.
- Pignanelli S, Shurdhi A, Delucca F, Donati M (2009). "Simultaneous use of direct and indirect diagnostic techniques in atypical respiratory infections from Chlamydophila pneumoniae and Mycoplasma pneumoniae". Journal of Clinical Laboratory Analysis. 23 (4): 206–9. doi:10.1002/jcla.20332. PMID 19623657.
- Zhan P, Suo LJ, Qian Q, Shen XK, Qiu LX, Yu LK, Song Y (March 2011). "Chlamydia pneumoniae infection and lung cancer risk: a meta-analysis". European Journal of Cancer. 47 (5): 742–7. doi:10.1016/j.ejca.2010.11.003. PMID 21194924.
- Mager DL (2006). "Bacteria and cancer: cause, coincidence or cure? A review". Journal of Translational Medicine. 4: 14. doi:10.1186/1479-5876-4-14. PMC 1479838. PMID 16566840.
- Littman AJ, Jackson LA, Vaughan TL (April 2005). "Chlamydia pneumoniae and lung cancer: epidemiologic evidence". Cancer Epidemiology, Biomarkers & Prevention. 14 (4): 773–8. doi:10.1158/1055-9965.EPI-04-0599. PMID 15824142.
- Kälvegren H, Bylin H, Leanderson P, Richter A, Grenegård M, Bengtsson T (August 2005). "Chlamydia pneumoniae induces nitric oxide synthase and lipoxygenase-dependent production of reactive oxygen species in platelets. Effects on oxidation of low density lipoproteins". Thrombosis and Haemostasis. 94 (2): 327–35. doi:10.1160/TH04-06-0360. PMID 16113822.
- Takaoka N, Campbell LA, Lee A, Rosenfeld ME, Kuo CC (February 2008). "Chlamydia pneumoniae infection increases adherence of mouse macrophages to mouse endothelial cells in vitro and to aortas ex vivo". Infection and Immunity. 76 (2): 510–4. doi:10.1128/IAI.01267-07. PMC 2223438. PMID 18070891.
- Mussa FF, Chai H, Wang X, Yao Q, Lumsden AB, Chen C (June 2006). "Chlamydia pneumoniae and vascular disease: an update". Journal of Vascular Surgery. 43 (6): 1301–7. doi:10.1016/j.jvs.2006.02.050. PMID 16765261.
- Sriram S, Stratton CW, Yao S, Tharp A, Ding L, Bannan JD, Mitchell WM (July 1999). "Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis". Annals of Neurology. 46 (1): 6–14. doi:10.1002/1531-8249(199907)46:1<6::AID-ANA4>3.0.CO;2-M. PMID 10401775.
- Hahn DL, Dodge RW, Golubjatnikov R (July 1991). "Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma". JAMA. 266 (2): 225–30. doi:10.1001/jama.266.2.225. PMID 2056624.
- Schmidt SM, Müller CE, Bruns R, Wiersbitzky SK (October 2001). "Bronchial Chlamydia pneumoniae infection, markers of allergic inflammation and lung function in children". Pediatric Allergy and Immunology. 12 (5): 257–65. doi:10.1034/j.1399-3038.2001.00042.x. PMID 11737672.
- Webley WC, Salva PS, Andrzejewski C, Cirino F, West CA, Tilahun Y, Stuart ES (May 2005). "The bronchial lavage of pediatric patients with asthma contains infectious Chlamydia". American Journal of Respiratory and Critical Care Medicine. 171 (10): 1083–8. doi:10.1164/rccm.200407-917OC. PMID 15735056.
- Hahn DL, McDonald R (October 1998). "Can acute Chlamydia pneumoniae respiratory tract infection initiate chronic asthma?". Annals of Allergy, Asthma & Immunology. 81 (4): 339–44. doi:10.1016/S1081-1206(10)63126-2. PMID 9809498.
- Arias I (April 2012). "Infectious agents associated with schizophrenia: a meta-analysis". Schizophr. Res. 136 (1–3): 128–136. doi:10.1016/j.schres.2011.10.026. PMID 22104141.
- Hahn DL (October 1995). "Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial". The Journal of Family Practice. 41 (4): 345–51. PMID 7561707.
- Hahn DL, Schure A, Patel K, Childs T, Drizik E, Webley W (2012). "Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity". PLoS One. 7 (4): e35945. Bibcode:2012PLoSO...735945H. doi:10.1371/journal.pone.0035945. PMC 3335830. PMID 22545149.
- Hahn DL, Plane MB, Mahdi OS, Byrne GI (June 2006). "Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma". PLoS Clinical Trials. 1 (2): e11. doi:10.1371/journal.pctr.0010011. PMC 1488900. PMID 16871333.
- Hahn DL, Grasmick M, Hetzel S, Yale S (2012). "Azithromycin for bronchial asthma in adults: an effectiveness trial". Journal of the American Board of Family Medicine. 25 (4): 442–59. doi:10.3122/jabfm.2012.04.110309. PMID 22773713.
- Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG (January 2008). "Clarithromycin targets neutrophilic airway inflammation in refractory asthma". American Journal of Respiratory and Critical Care Medicine. 177 (2): 148–55. CiteSeerX 10.1.1.318.5663. doi:10.1164/rccm.200707-1134OC. PMID 17947611.
- Von HL, Vasankari T, Liippo K, Wahlström E, Puolakkainen M (2002). "Chlamydia pneumoniae and severity of asthma". Scandinavian Journal of Infectious Diseases. 34 (1): 22–7. doi:10.1080/00365540110077155. PMID 11874160.
- Cho YS, Kim TB, Lee TH, Moon KA, Lee J, Kim YK, Lee KY, Moon HB (December 2005). "Chlamydia pneumoniae infection enhances cellular proliferation and reduces steroid responsiveness of human peripheral blood mononuclear cells via a tumor necrosis factor-alpha-dependent pathway". Clinical and Experimental Allergy. 35 (12): 1625–31. doi:10.1111/j.1365-2222.2005.02391.x. PMID 16393329.
- Reiter J, Demirel N, Mendy A, Gasana J, Vieira ER, Colin AA, Quizon A, Forno E (August 2013). "Macrolides for the long-term management of asthma--a meta-analysis of randomized clinical trials". Allergy. 68 (8): 1040–9. doi:10.1111/all.12199. PMID 23895667.