Cerdulatinib
Cerdulatinib is a small molecule SYK/JAK kinase inhibitor in development for treatment of hematological malignancies.[1] It has lowest nM IC50 values against TYK2, JAK1, JAK2, JAK3, FMS, and SYK.[2]
 | |
| Clinical data | |
|---|---|
| Other names | PRT062070, PRT2070, DMVT-502 |
| Routes of administration | By mouth |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
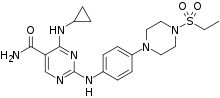
| Formula | C20H27N7O3S |
| Molar mass | 445.54 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
It is being developed by Portola Pharmaceuticals; in September 2018 the FDA granted orphan drug status to cerdulatinib for the treatment of peripheral T-cell lymphoma (PTCL).[3]
See also
References
- Liu, Delong; Mamorska-Dyga, Aleksandra (2017-07-28). "Syk inhibitors in clinical development for hematological malignancies". Journal of Hematology & Oncology. 10 (1): 145. doi:10.1186/s13045-017-0512-1. ISSN 1756-8722. PMC 5534090. PMID 28754125.
- Coffey, Greg; Betz, Andreas; DeGuzman, Francis; Pak, Yvonne; Inagaki, Mayuko; Baker, Dale C.; Hollenbach, Stanley J.; Pandey, Anjali; Sinha, Uma (2014). "The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and B-cell cancer". The Journal of Pharmacology and Experimental Therapeutics. 351 (3): 538–548. doi:10.1124/jpet.114.218164. ISSN 1521-0103. PMID 25253883.
- "Investors - News Release - Portola Pharmaceuticals, Inc". phx.corporate-ir.net. September 25, 2018.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.