Buruli ulcer
Buruli ulcer is an infectious disease caused by Mycobacterium ulcerans.[3] The early stage of the infection is characterised by a painless nodule or area of swelling.[3] This nodule can turn into an ulcer.[3] The ulcer may be larger inside than at the surface of the skin,[4] and can be surrounded by swelling.[4] As the disease worsens, bone can be infected.[3] Buruli ulcers most commonly affect the arms or legs;[3] fever is uncommon.[3]
| Buruli ulcer | |
|---|---|
| Other names | Bairnsdale ulcers, Searls ulcer, Daintree ulcer[1][2] |
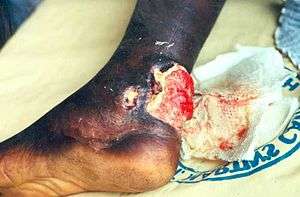 | |
| Buruli ulcer on the ankle of a person from Ghana. | |
| Specialty | Infectious disease |
| Symptoms | Area of swelling that becomes an ulcer[3] |
| Causes | Mycobacterium ulcerans[3] |
| Treatment | Rifampicin and streptomycin[3] |
| Frequency | ~ 2,000 cases reported (2015)[3] |
M. ulcerans releases a toxin known as mycolactone, which decreases immune system function and results in tissue death.[3] Bacteria from the same group also cause tuberculosis and leprosy (M. tuberculosis and M. leprae, respectively).[3] How the disease is spread is not known.[3] Sources of water may be involved in the spread.[4] As of 2018 there is no effective vaccine.[5][6] The Bacillus Calmette–Guérin (BCG) vaccine has demonstrated limited protection.[3]
If people are treated early, antibiotics for eight weeks are effective in 80% of cases.[3][7] The treatment often includes the medications rifampicin and streptomycin.[3] Clarithromycin or moxifloxacin are sometimes used instead of streptomycin.[3] Other treatments may include cutting out the ulcer.[3][8] After the infection heals, the area typically has a scar.[6]
About 2,000 cases are reported a year.[5] Buruli ulcers occur most commonly in rural sub-Saharan Africa and Australia with fewer cases in South America and the Western Pacific.[5] Children are most commonly infected in Africa, while adults are most commonly affected in Australia.[5] Cases have been reported in 33 countries.[5] The disease also occurs in animals other than humans, though no link between animal and human infection has been established.[9] Albert Ruskin Cook was the first to describe buruli ulcers in 1897.[4] It is classified as a neglected tropical disease.[10]
Signs and symptoms
The infection, in most instances, presents as a painless lump or swelling and is often unaccompanied by fever.[3] In southern Australia, the presentation is more often as a pimple in the skin (dermis) rather than under it and may be confused with an insect bite.[3] The infection is mostly in the limbs,[3] most often in exposed areas, but not on the hands or feet. In children, all areas may be involved, including the face or abdomen. The oedematous form of infection produces diffuse swelling of a limb, which, unlike the papule or nodule, can be painful and accompanied by low-grade fever.[7] Infection may follow physical trauma, often minor trauma such as a small scratch.[11]
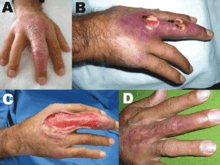
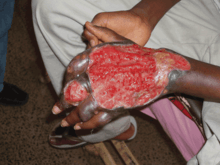 A typical Buruli ulcer on the left hand of a 17-year-old boy in Nigeria
A typical Buruli ulcer on the left hand of a 17-year-old boy in Nigeria Healed Buruli ulcer lesions in a Ghanaian woman
Healed Buruli ulcer lesions in a Ghanaian woman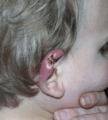 Ear of an 18-month-old with confirmed Buruli ulcer
Ear of an 18-month-old with confirmed Buruli ulcer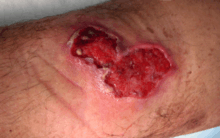 Buruli ulcer in a long-term traveler to Senegal
Buruli ulcer in a long-term traveler to Senegal
Cause
The disease is caused by Mycobacterium ulcerans.[3] It often occurs in close proximity to water bodies,[11] but no specific activities that bring people into contact with water have been identified (i.e. fetching of water, fishing, rice farming, washing, bathing, etc.). The mode of transmission of Buruli ulcer is not entirely known. Recent evidence suggests insects may be involved in the transmission of the infection.[12] These insects are aquatic bugs belonging to the genus Naucoris (family Naucoridae) and Diplonychus (family Belostomatidae). Trauma is probably the most frequent means by which M. ulcerans is introduced into the skin from surface contamination.[13] The initial trauma can be a mild skin wound such as scratch.[14] Other studies have suggested aerosol spread but these are not proven.[15] In Australia, animals such as koalas and possums are naturally infected.[16][17] Epidemiological evidence has not clearly supported person-to-person transmission. However, Muelder & Nourou found that 10 out of 28 patients had relatives who had also had the disease, and cautioned against the dismissal of person-to-person transmission.[18] Given the number of patients who shed large numbers of bacilli from their wounds and live in very close contact with relatives, more cases should have been observed. The cases reported by Muelder & Nourou could perhaps have been exposed to a common source of infection, and there might also be genetic component to sensitivity to the disease.
After considering the various suspected agents, Portaels et al. proposed the hypothesis that human beings, as well as domestic and wild animals, could be contaminated or infected by biting insects such as water bugs.[19] Aquatic bugs are cosmopolite insects found throughout temperate and tropical regions especially rich in freshwater. They represent about 10% of all species of Hemiptera associated with water and belong to two series of the suborder Heteroptera: the Nepomorpha, which include four superfamilies whose members spend most of their time under water, and the Naucoroidea, which include a single family, the Naucoridae, whose members are commonly termed creeping water bugs.
Whether found in temperate countries like France or tropical ones like Ivory Coast, aquatic bugs exhibit the same way of life, preying, according to their size, on mollusks, snails, young fish, and the adults and larvae of other insects that they capture with their raptorial front legs and bite with their rostrum. These insects can inflict painful bites on humans as well. In the Ivory Coast, where Buruli ulcer is endemic, the water bugs are present in swamps and rivers, where human activities such as farming, fishing, and bathing take place. Present findings[20] describing the experimental transmission of M. ulcerans from water bugs to mice are in good agreement with the possibility of this mode of transmission to humans by bites.
Also in strong support of this hypothesis was the localization of M. ulcerans within the salivary glands of Naucoridae.[20] Local physiological conditions of this niche appear to fit the survival and the replication needs of M. ulcerans but not those of other mycobacteria. Surprisingly, infiltration of the salivary glands of Naucoridae by M. ulcerans does not seem to be accompanied by any tissue damage similar to the ulcerative skin lesions developed by bitten individuals and mediated by the cytotoxic activity of the mycolactone[21] and other toxins produced by M. ulcerans.[22] The inactivation of the latter toxins could be the result of salivary enzymatic activities, which remain to be determined.
Mycobacterium ulcerans was first cultivated and characterized from the environment in 2008.[23]
Pathology
The disease is primarily an infection of subcutaneous fat, resulting in a focus of necrotic (dead) fat containing myriads of the mycobacteria in characteristic spherules formed within the dead fat cells. Skin ulceration is a secondary event.
M. ulcerans releases a lipid toxin, mycolactone, which functions as an immune suppressant, necrotising agent and activator of cellular death.[24][25]
Healing may occur spontaneously but more often the disease is slowly progressive with further ulceration, granulation, scarring, and contractures. Satellite infection may occur with other nodules developing and infection may occur into bone. Although seldom fatal, the disease results in considerable morbidity and deformity.
Th1-mediated immune responses are protective against M. ulcerans infection, whereas Th2-mediated responses are not.
Diagnosis
The diagnosis of Buruli ulcer is usually based on the characteristic appearance of the ulcer in an endemic area. If there is any doubt about the diagnosis, then PCR using the IS2404 target is helpful, but this is not specific for M. ulcerans. The Ziehl-Neelsen stain is only 40–80% sensitive, and culture is 20–60% sensitive. Simultaneous use of multiple methods may be necessary to make the diagnosis.[26]
Prevention
There is no specific vaccine for Myocobacterium ulcerans.[6] The Bacillus Calmette-Guérin vaccine may offer temporary protection.[3]
Treatment
If treated early, antibiotics for eight weeks are effective in 80% of people.[3] This often includes the medications rifampicin and streptomycin.[3] Clarithromycin or moxifloxacin are sometimes used instead of streptomycin.[3]
Treatment may also include cutting out the ulcer.[8] This may be a minor operation and very successful if undertaken early. Advanced disease may require prolonged treatment with extensive skin grafting. Surgical practice can be dangerous in the developing countries where the disease is common.
Epidemiology
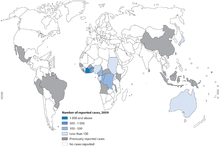
The infection occurs in well-defined areas throughout the world, mostly tropical areas — in several areas in Australia, in Uganda, in several countries in West Africa, in Central and South America, in southeast Asia and New Guinea. It is steadily rising as a serious disease, especially in West Africa and underdeveloped countries, where it is the third leading cause of mycobacterial infection in healthy people, after tuberculosis and leprosy.
The disease is more likely to occur where there have been environmental changes such as the development of water storages, sand mining, and irrigation.
Buruli ulcer is currently endemic in Benin, Côte d'Ivoire, Ghana, Guinea, Liberia, Nigeria, Sierra Leone and Togo.[27] In Ghana, 1999 data indicated that the prevalence rate of the disease in the Ga West District was 87.7 per 100,000, higher than the estimated national prevalence rate at 20.7 per 100,000 generally, and 150.8 per 100,000 in the most disease-endemic districts.[28]
Geographical distribution
Buruli ulcer has been reported from at least 32 countries around the world, mostly in tropical areas:
- West Africa: Benin, Burkina Faso, Côte d'Ivoire, Ghana, Liberia, Nigeria, Togo, Guinea, Sierra Leone.
- Other African Countries: Angola, Cameroon, Congo, Democratic Republic of Congo, Equatorial Guinea, Gabon, Sudan, Uganda.
- Western Pacific: Australia, Papua New Guinea, Kiribati.
- Americas: French Guiana, Mexico, Peru, Suriname.
- Asia: China, Malaysia, Japan.
In several of these countries, the disease is not considered to be a public health problem, hence the current distribution and the number of cases are not known. Possible reasons include:
- the distribution of the disease is often localized in certain parts of endemic countries;
- Buruli ulcer is not a notifiable disease
- In most places where the disease occurs, patients receive care from private sources such as voluntary mission hospitals and traditional healers. Hence the existence of the disease may not come to the attention of the ministries of health.
It most commonly occurs in Africa: Congo and Cameroon in Central Africa, Côte d'Ivoire, Ghana and Benin in West Africa. Some Southeast Asian countries (Papua New Guinea) and Australia have major foci, and there have been a few patients reported from South America (French Guyana and Surinam) and Mexico. Focal outbreaks have followed flooding, human migrations,[29] and man-made topographic modifications such as dams and resorts. Deforestation and increased basic agricultural activities may significantly contribute to the recent marked increases in the incidence of M. ulcerans infections, especially in West Africa, where the disease is rapidly emerging.
Race, age and sex
Buruli ulcer commonly affects poor people in remote rural areas with limited access to health care. The disease can affect all age groups, although children under the age of 15 years (range 2–14 years) are predominantly affected. There are no sex differences in the distribution of cases among children. Among adults, some studies have reported higher rates among women than males (Debacker et al. accepted for publication). No racial or socio-economic group is exempt from the disease. Most ulcers occur on the extremities; lesions on the lower extremities are almost twice as common as those on the upper extremities. Ulcers on the head and trunk accounted for less than 8% of cases in one large series.[30]
History
James Augustus Grant, in his book A Walk across Africa (1864), describes how his leg became grossly swollen and stiff with later a copious discharge. This was almost certainly the severe edematous form of the disease, and is the first known description of the infection. Buruli ulcer disease was identified in 1897 by Sir Albert Cook, a British physician, at Mengo Hospital in Kampala, Uganda. The disease was named after Buruli County in Uganda (now called Nakasongola District), because of the many cases that occurred there in the 1960s.[31] The incidence of the disease has recently been rising in tropical Africa and in certain parts of Australia.
A detailed description of the disease was written in 1948 by Professor Peter MacCallum and his colleagues, who were treating patients from the Bairnsdale district, in the Gippsland region in eastern Victoria, Australia. MacCallum and his team were the first to identify Mycobacterium ulcerans as the pathogen causing the condition. In Australia it is also known as Bairnsdale or Daintree ulcer.
In March 2008, researchers announced the first isolation of M. ulcerans from the environment.[23] This suggested that the disease might be transmitted via contact with the environment rather than person to person.[23] The entire genome of M. ulcerans has been sequenced.[32]
Certain types of clay have historically been used in an attempt to treat the condition.[33]
References
- James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. p. 340. ISBN 978-0-7216-2921-6.
- Lavender CJ, Senanayake SN, Fyfe JA, et al. (January 2007). "First case of Mycobacterium ulcerans disease (Bairnsdale or Buruli ulcer) acquired in New South Wales". Med. J. Aust. 186 (2): 62–3. doi:10.5694/j.1326-5377.2007.tb00801.x. PMID 17223764. Archived from the original on 2011-04-05.
- "Buruli ulcer (Mycobacterium ulcerans infection) Fact sheet N°199". World Health Organization. June 2013. Archived from the original on 27 February 2014. Retrieved 23 February 2014.
- Nakanaga, K; Yotsu, RR; Hoshino, Y; Suzuki, K; Makino, M; Ishii, N (2013). "Buruli ulcer and mycolactone-producing mycobacteria". Japanese Journal of Infectious Diseases. 66 (2): 83–8. doi:10.7883/yoken.66.83. PMID 23514902.
- "Buruli ulcer (Mycobacterium ulcerans infection) Fact sheet N°199". World Health Organization. April 2018. Retrieved 17 April 2018.
- Einarsdottir T, Huygen K (November 2011). "Buruli ulcer". Hum Vaccin. 7 (11): 1198–203. doi:10.4161/hv.7.11.17751. PMID 22048117.
- "Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers". World Health Organization. 2012. Retrieved 17 April 2018.
- Sizaire V, Nackers F, Comte E, Portaels F (2006). "Mycobacterium ulcerans infection: control, diagnosis, and treatment" (PDF). Lancet Infect Dis. 6 (5): 288–296. doi:10.1016/S1473-3099(06)70464-9. hdl:10144/17727. PMID 16631549.
- "Buruli Ulcer: Transmission". Centers for Disease Control and Prevention. 26 January 2015. Retrieved 17 April 2018.
- "Neglected Tropical Diseases". cdc.gov. June 6, 2011. Archived from the original on 4 December 2014. Retrieved 28 November 2014.
- "Buruli ulcer - Diagnosis of Mycobacterium ulcerans disease". World Health Organization. Retrieved 2018-04-17.
- Portaels, F.; Elsen, P.; Guimaraes-Peres, A.; Fonteyne, P.A.; Meyers, W.M. (1999). "Insects in the transmission of Mycobacterium ulcerans infection". Lancet. 353 (9157): 986. doi:10.1016/S0140-6736(98)05177-0. PMID 10459918.
- Stienstra, Y.; van der Graaf, T.A.; te Meerman, G.J.; The, T.H.; de Leij, L.F.; van der Werf, T.S. (2001). "Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors". Trop Med Int Health. 6 (7): 554–562. doi:10.1046/j.1365-3156.2001.00746.x. PMID 11469950.
- Meyers, W.M.; Shelly, W.M.; Connor, D.H.; Meyers, E.K. (1974). "Human Mycobacterium ulcerans infections developing at sites of trauma to skin". Am J Trop Med Hyg. 23 (5): 919–923. doi:10.4269/ajtmh.1974.23.919. PMID 4451232.
- Veitch MG, Johnson PD, Flood PE, Leslie DE, Street AC, Hayman JA (December 1997). "A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island". Epidemiol. Infect. 119 (3): 313–8. doi:10.1017/S0950268897008273. PMC 2809003. PMID 9440434.
- Mitchell PJ, Jerrett IV, Slee KJ (1984). "Skin ulcers caused by Mycobacterium ulcerans in koalas near Bairnsdale, Australia". Pathology. 16 (3): 256–260. doi:10.3109/00313028409068533. PMID 6514393.
- Flood P, Street A, O'Brien P, Hayman J (February 1994). "Mycobacterium ulcerans infection on Phillip Island, Victoria". Med. J. Aust. 160 (3): 160. doi:10.5694/j.1326-5377.1994.tb126569.x. PMID 8295586.
- Muelder, K.; A. Nourou (1990). "Buruli ulcer in Benin". Lancet. 336 (8723): 1109–1111. doi:10.1016/0140-6736(90)92581-2. PMID 1977990.
- Portaels F, Chemlal K, Elsen P, et al. (April 2001). "Mycobacterium ulcerans in wild animals". Rev. - Off. Int. Epizoot. 20 (1): 252–64. doi:10.20506/rst.20.1.1270. PMID 11288515.
- Marsollier, L.; R. Robert; J. Aubry; J. P. Saint André; H. Kouakou; P. Legras; A. L. Manceau; C. Mahaza & B. Carbonnelle (2002). "Aquatic insects as a vector for Mycobacterium ulcerans". Appl Environ Microbiol. 68 (9): 4623–8. doi:10.1128/AEM.68.9.4623-4628.2002. PMC 124085. PMID 12200321.
- George, K. M.; L. Pascopella; D. M. Welty & P. L. C. Small (2000). "A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in Guinea pig ulcers and tissue culture cells". Infect Immun. 68 (2): 877–883. doi:10.1128/IAI.68.2.877-883.2000. PMC 97217. PMID 10639458.
- Dobos, K.M.; Small, P.L.; Deslauriers, M.; Quinn, F.D.; King, C.H. (2001). "Mycobacterium ulcerans cytotoxicity in an adipose cell". Infect Immun. 69 (11): 7182–6. doi:10.1128/IAI.69.11.7182-7186.2001. PMC 100123. PMID 11598099.
- Portaels F, Meyers WM, Ablordey A, Castro AG, Chemlal K, de Rijk P, Elsen P, Fissette K, Fraga AG, Lee R, Mahrous E, Small PL, Stragier P, Torrado E, Van Aerde A, Silva MT, Pedrosa J (2008). "First Cultivation and Characterization of Mycobacterium ulcerans from the Environment". PLoS Negl Trop Dis. 2 (3): e178. doi:10.1371/journal.pntd.0000178. PMC 2268003. PMID 18365032. Lay summary – EurekAlert! (2008-03-25).

- van der Werf TS, Stinear T, Stienstra Y, van der Graaf WT, Small PL (September 2003). "Mycolactones and Mycobacterium ulcerans disease". Lancet. 362 (9389): 1062–4. doi:10.1016/S0140-6736(03)14417-0. PMID 14522538.
- Adusumilli S, Mve-Obiang A, Sparer T, Meyers W, Hayman J, Small PL (September 2005). "Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo". Cell. Microbiol. 7 (9): 1295–304. doi:10.1111/j.1462-5822.2005.00557.x. PMID 16098217.
- Herbinger K-H; Adjei O; Awua‐Boateng N-Y; et al. (2009). "Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease". Clin Infect Dis. 48 (8): 1055–64. doi:10.1086/597398. PMID 19275499.
- WHO, (2000) Buruli ulcer: Mycobacterium ulcerans infection. Geneva
- Amofah G, Bonsu F, Tetteh C, et al. (February 2002). "Buruli ulcer in Ghana: results of a national case search". Emerging Infect. Dis. 8 (2): 167–70. doi:10.3201/eid0802.010119. PMC 2732443. PMID 11897068.
- Uganda Buruli Group (1971). "Epidemiology of Mycobacterium ulcerans infection (Buruli ulcer) at Kinyara, Uganda". Trans R Soc Trop Med Hyg. 65 (6): 763–775. doi:10.1016/0035-9203(71)90090-3. PMID 5157438.
- Marston, B.J.; Diallo, M.O.; Horsburgh jr., C.R.; Diomande, I.; Saki, M.Z.; Kanga, J.M.; Patrice, G.; Lipman, H.B.; Ostroff, S.M.; Good, R.C. (1995). "Emergence of Buruli Ulcer disease in the Daloa region of Côte d'Ivoire". Am J Trop Med Hyg. 52 (3): 219–224. doi:10.4269/ajtmh.1995.52.219. PMID 7694962.
- "Buruli ulcer disease -Mycobacterium ulcerans infection". Health Topics A TO Z. Archived from the original on 2010-12-04. Retrieved 2010-12-24.
- Yoshida M, Nakanaga K, Ogura Y, Toyoda A, Ooka T, Kazumi Y, Mitarai S, Ishii N, Hayashi T, Hoshino Y (2016). "Complete Genome Sequence of Mycobacterium ulcerans subsp. shinshuense". Genome Announc. 4 (5). doi:10.1128/genomeA.01050-16. PMC 5043562. PMID 27688344.
- "New answer to MRSA, other 'superbug' infections: clay minerals? | NSF - National Science Foundation". www.nsf.gov. Retrieved 18 April 2018.
- Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. Chapter 74. ISBN 978-1-4160-2999-1.
- Rudolf, Zdenek Hubálek, Ivo (2011). Microbial zoonoses and sapronoses. Dordrecht: Springer. p. 258. ISBN 9789048196579. Archived from the original on 2017-09-08.
- Yann A. Meunier (2014). Tropical diseases : a practical guide for medical practitioners and students. Contributions from Michael Hole, Takudzwa Shumba & B.J. Swanner. Oxford: Oxford University Press. p. 167. ISBN 9780199997909. Archived from the original on 2017-09-08.
- Medical Journal of Australia. 2. Australasian Medical Publishing Company. 1966. p. 926.
External links
| Classification | |
|---|---|
| External resources |
- World Health Organization buruli ulcer page
- "Mycobacterium ulcerans". NCBI Taxonomy Browser. 1809.
- Merritt, R. W.; Walker, E. D.; Small, P. L. C.; Wallace, J. R.; Johnson, P. D. R.; Benbow, M. E.; Boakye, D. A. (2010). Phillips, Richard O (ed.). "Ecology and Transmission of Buruli Ulcer Disease: A Systematic Review". PLoS Neglected Tropical Diseases. 4 (12): e911. doi:10.1371/journal.pntd.0000911. PMC 3001905. PMID 21179505.
