Borrelia
Borrelia is a genus of bacteria of the spirochete phylum.[1] It causes Lyme disease, also called Lyme borreliosis, a zoonotic, vector-borne disease transmitted primarily by ticks and by lice, depending on the species of bacteria.[2] The genus is named after French biologist Amédée Borrel (1867–1936), who first documented the distinction between a species of Borrelia, B. anserina, and the other known type of spirochete at the time, Treponema pallidum.[3] This bacterium must be viewed using dark-field microscopy,[4] which make the cells appear white against a dark background. Borrelia species are grown in Barbour-Stoenner-Kelly medium.[4] Of 52 known species of Borrelia, 21 are members of the Lyme disease group, 29 belong to the relapsing fever group, and two are members of a genetically distinct third group typically found in reptiles.[3] The Lyme disease group has been moved to their own genus, Borelliella, but this change is not yet widely accepted.[3] This bacterium uses hard and soft ticks and lice as vectors.[5][6] Testing for the presence of the bacteria in a human includes two-tiered serological testing, including immunoassays and immunoblotting.[7]
| Borrelia | |
|---|---|
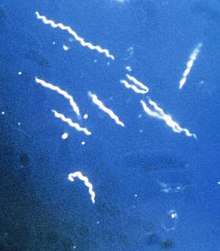 | |
| Borrelia burgdorferi the causative agent of Lyme disease (borreliosis) magnified 400 times | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Spirochaetes |
| Order: | Spirochaetales |
| Family: | Spirochaetaceae |
| Genus: | Borrelia Swellengrebel 1907 |
| Species | |
| |
Biology
Borrelia species are members of the Spirochaetaceae family, so present the characteristic spirochete (spiral) shape. Most species are obligate anaerobes, although some are aerotolerant.[8] Borrelia species have an outer membrane that contains a substance similar to lipopolysaccharides, an inner membrane, and a layer of peptidoglycan in a periplasmic space, which classifies them as Gram-negative.[4] However, this result is not easily visualized using Gram staining.[4] They are typically 20–30 μm long and 0.2–0.3 μm wide.[4]
Spirochetes move using axial filaments called endoflagella in their periplasmic space.[4] The filaments rotate in this space, between the outer membrane and the peptidoglycan layer, propelling the bacterium forward in a corkscrew-like motion.[4] The outer membrane of Borrelia species contains outer surface proteins (Osp) that play a role in their virulence.[4]
Phylogeny
The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature (LPSN)[1] and National Center for Biotechnology Information (NCBI)[1] and the phylogeny is based on 16S rRNA-based LTP release 111 by the All-Species Living Tree Project.[1]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Notes:
♦ Type strain lost or not available
♠ Strains found at the NCBI, but not listed in the LSPN
♥ Strains neither lodged at NCBI nor listed in the LPSN
Vectors
Ticks
Hard ticks of the family Ixodidae are common vectors of Borellia bacteria[9] and are the only type of ticks shown to transmit Lyme disease bacteria to humans.[10]
| Region | Tick species | Common name |
|---|---|---|
| East and Midwest (US) | Ixodes scapularis | Black-legged tick, deer tick |
| Pacific Coast (US) | Ixodes pacificus | Western black-legged tick |
| Europe | Ixodes ricinus | Sheep tick |
| Asia | Ixodes persulcatus | Taiga tick |
Other species are carried by soft ticks. The soft tick Ornithodoros carries the species of Borellia that cause relapsing fever.[6] Another species, B. anserina, is carried by the soft tick Argas.[3] Inside the ticks, the bacteria grow in the midgut and then travel to the salivary glands to be transmitted to a new host.[10] Ticks can spread the bacteria to each other when co-feeding.[9] If an animal has been infected by a tick and then is bitten by a second tick, the second tick can become infected.[11] The bacteria are most commonly transmitted to humans through ticks in the nymph stage of development, because they are smaller and less likely to be noticed and removed.[11] The ticks must have around 36 to 48 hours of contact with a host to successfully transmit the bacteria.[11]
Lice
Lice that feed on infected humans acquire the Borrelia organisms that then multiply in the hemolymph and gut of the lice.[6] When an infected louse feeds on an uninfected human, the organism gains access when the victim crushes the louse or scratches the area where the louse is feeding.[6] The U. S. Centers for Disease Control and Prevention reported that no credible evidence shows that lice can carry Borrelia.[11]
Lyme disease
Of the 52 known species of Borrelia, 21 are known to cause Lyme disease or borreliosis and are transmitted by ticks.[3] The major Borrelia species causing Lyme disease are Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii.[12] All species that cause Lyme disease are referred to collectively as B. burgdorferi sensu lato,[7] while B. burgdorferi itself is specified as B. burgdorferi sensu stricto.[7][10] B. burgdorferi was previously believed to be the only species to cause Lyme disease in the US, with the other two existing only in Europe and Asia, but a new species called B. mayonii has caused Lyme disease in the US, as well.[12]
Relapsing fever
Relapsing fever (RF) borreliosis often occurs with severe bacteremia.[13] Twenty-five species of Borrelia are known to cause relapsing fever.[14] While most species use the soft tick family Argasidae as their vector, some outliers live in hard ticks or lice.[14] Relapsing fever can be spread epidemically through lice or endemically through ticks.[6]
B. recurrentis, a common species underlying relapsing fever, is transmitted by the human body louse; no other animal reservoir of B. recurrentis is known.[6] B. recurrentis infects the person via mucous membranes and then invades the bloodstream.[6]
Other tick-borne relapsing infections are acquired from other species, such as B. hermsii, B. parkeri, or B. miyamotoi,[15] which can be spread from rodents, and serve as a reservoir for the infection, via a tick vector. B. hermsii and B. recurrentis cause very similar diseases, although the disease associated with B. hermsii has more relapses and is responsible for more fatalities, while the disease caused by B. recurrentis has longer febrile and afebrile intervals and a longer incubation period.
Diagnosis
Direct tests include culture of Borrelia from skin, blood, or cerebrospinal fluid (CSF), and detection of genetic material by polymerase chain reaction in skin, blood, or synovial fluid. Two-tiered serological testing is performed for differential diagnosis of Borrelia infection. The first-tier tests detect specific antibodies (IgM and IgG together or separately) and include enzyme-linked immunoassays (e.g. ELISAs) and immunofluorescent assays. Positive results for first-tier tests are confirmed using second-tier testing. The second tier consists of standardized immunoblotting, either by using Western blots or blots striped with diagnostically important purified antigens. Positive results for second-tier tests are confirmatory for the presence of Borrelia infection.[7][16] Spirochetes can also be seen using Wright-stained blood smears.[6]
References
- Parte AC (January 2014). "LPSN—list of prokaryotic names with standing in nomenclature". Nucleic Acids Research. 42 (Database issue): D613–6. doi:10.1093/nar/gkt1111. PMC 3965054. PMID 24243842.
- Samuels DS, Radolf JD, eds. (2010). Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press. ISBN 978-1-904455-58-5.
- Cutler SJ, Ruzic-Sabljic E, Potkonjak A (February 2017). "Emerging borreliae – Expanding beyond Lyme borreliosis" (PDF). Molecular and Cellular Probes. 31: 22–27. doi:10.1016/j.mcp.2016.08.003. PMID 27523487.
- Todar K (2006). Todar's Online Textbook of Bacteriology. University of Wisconsin-Madison Department of Bacteriology. OCLC 803733454.
- Shapiro ED (2008), "Principles and Practice of Pediatric Infectious Disease", Pediatrics in Review, Elsevier, 35 (12): 940–944, doi:10.1542/pir.35-12-500, ISBN 9780702034688, PMC 5029759, PMID 25452659
- Petri WA (2012). "330 - Relapsing Fever and Other Borrelia Infections". Goldman's Cecil Medicine. 2 (Twenty-Fourth ed.). pp. 1935–1937. doi:10.1016/b978-1-4377-1604-7.00330-4. ISBN 9781437716047.
- Marques AR (June 2015). "Laboratory diagnosis of Lyme disease: advances and challenges". Infectious Disease Clinics of North America. 29 (2): 295–307. doi:10.1016/j.idc.2015.02.005. PMC 4441761. PMID 25999225.
- De Martino SJ, Sordet C, Piémont Y, Ruzic-Sabljic E, Thaddée Vetter M, Monteil H, Sibilia J, Jaulhac B (October 2006). "Enhanced culture of Borrelia garinii and Borrelia afzelii strains on a solid BSK-based medium in anaerobic conditions". Research in Microbiology. 157 (8): 726–9. doi:10.1016/j.resmic.2006.05.002. PMID 16814991.
- Heylen D, Lasters R, Adriaensen F, Fonville M, Sprong H, Matthysen E (March 2019). "Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area". The Science of the Total Environment. 670: 941–949. Bibcode:2019ScTEn.670..941H. doi:10.1016/j.scitotenv.2019.03.235. PMID 30921726.
- Tilly K, Rosa PA, Stewart PE (June 2008). "Biology of infection with Borrelia burgdorferi". Infectious Disease Clinics of North America. 22 (2): 217–34, v. doi:10.1016/j.idc.2007.12.013. PMC 2440571. PMID 18452798.
- "Transmission | Lyme Disease | CDC". www.cdc.gov. 2019-02-06. Retrieved 2019-04-03.
- "Borrelia mayonii | Ticks | CDC". www.cdc.gov. 2019-01-10. Retrieved 2019-04-03.
- Guo BP, Teneberg S, Münch R, Terunuma D, Hatano K, Matsuoka K, Angström J, Borén T, Bergström S (November 2009). "Relapsing fever Borrelia binds to neolacto glycans and mediates rosetting of human erythrocytes". Proceedings of the National Academy of Sciences of the United States of America. 106 (46): 19280–5. Bibcode:2009PNAS..10619280G. doi:10.1073/pnas.0905470106. PMC 2771742. PMID 19884498.
- Wang G (2015). "Chapter 104 - Borrelia burgdorferi and Other Borrelia Species". Molecular Medical Microbiology. 3 (Second ed.). pp. 1867–1909. doi:10.1016/b978-0-12-397169-2.00104-9.
- McNeil D (19 September 2011). "New Tick-Borne Disease Is Discovered". The New York Times. pp. D6. Retrieved 20 September 2011.
- Johnson BJ, Robbins KE, Bailey RE, Cao BL, Sviat SL, Craven RB, Mayer LW & Dennis DT (August 1996). "Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting". The Journal of Infectious Diseases. 174 (2): 346–53. doi:10.1093/infdis/174.2.346. PMID 8699065.
Further reading
- Samuels DS, Radolf JD, eds. (2010). Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press. ISBN 978-1-904455-58-5.
- Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO (2007). "SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB". Nucleic Acids Research. 35 (21): 7188–96. doi:10.1093/nar/gkm864. PMC 2175337. PMID 17947321.
- Guo BP, Teneberg S, Münch R, Terunuma D, Hatano K, Matsuoka K, Angström J, Borén T, Bergström S (November 2009). "Relapsing fever Borrelia binds to neolacto glycans and mediates rosetting of human erythrocytes". Proceedings of the National Academy of Sciences of the United States of America. 106 (46): 19280–5. Bibcode:2009PNAS..10619280G. doi:10.1073/pnas.0905470106. PMC 2771742. PMID 19884498.
External links
- Borrelia genomes and related information at PATRIC, a Bioinformatics Resource Center funded by NIAID
- Borrelia Microbe Wiki Page
- NCBI Borrelia Taxonomy Browser