Bempedoic acid
Bempedoic acid is a drug under development by Esperion Therapeutics for the treatment of hypercholesterolemia.[2] In a study, it reduced LDL cholesterol by about 20 mg/dl compared to placebo and had no more side effects than placebo, although a higher percentage of drug receiving patients dropped out of the study because of side effects (11% vs. 7% under placebo).[3] As of March 2019, its effects on cardiovascular morbidity and mortality have not been determined; but studies are under way.[4]
| Clinical data | |
|---|---|
| Other names | ESP-55016, ETC-1002 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 15–24 hrs[1] |
| Identifiers | |
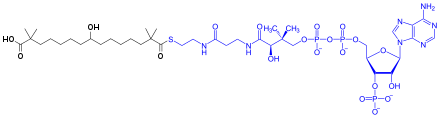
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.238.679 |
| Chemical and physical data | |
| Formula | C19H36O5 |
| Molar mass | 344.492 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mechanism of action
Bempedoic acid is a prodrug. It is activated to the thioester with coenzyme A by the enzyme SLC27A2 in the liver.[1] The activated substance inhibits ATP citrate lyase, which is involved in the liver's biosynthesis of cholesterol upstream of HMG-CoA reductase, the enzyme that is blocked by statins.[3][4]
The substance also activates AMP-activated protein kinase, but this effect is likely not relevant in humans.[1]
References
- Bilen, Ozlem; Ballantyne, Christie M. (2016). "Bempedoic Acid (ETC-1002): An Investigational Inhibitor of ATP Citrate Lyase". Current Atherosclerosis Reports. 18 (10): 61. doi:10.1007/s11883-016-0611-4. PMC 5035316. PMID 27663902.
- Clinical trial number NCT02988115 for "Evaluation of the Efficacy and Safety of Bempedoic Acid (ETC-1002) in Patients With Hyperlipidemia and Statin Intolerant (CLEAR Serenity)" at ClinicalTrials.gov
- Ray, Kausik K.; Bays, Harold E.; Catapano, Alberico L.; Lalwani, Narendra D.; Bloedon, Leanne T.; Sterling, Lulu R.; Robinson, Paula L.; Ballantyne, Christie M.; CLEAR Harmony Trial (2019). "Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol". New England Journal of Medicine. 380 (11): 1022–1032. doi:10.1056/NEJMoa1803917. PMID 30865796.
- "Bempedoic Acid". Esperion Therapeutics. Retrieved 2019-03-15.