Basal ganglia disease
Basal ganglia disease is a group of physical problems that occur when the group of nuclei in the brain known as the basal ganglia fail to properly suppress unwanted movements or to properly prime upper motor neuron circuits to initiate motor function.[1] Research indicates that increased output of the basal ganglia inhibits thalamocortical projection neurons. Proper activation or deactivation of these neurons is an integral component for proper movement. If something causes too much basal ganglia output, then the Ventral Anterior (VA) and Ventral Lateral (VL) thalamocortical projection neurons become too inhibited and one cannot initiate voluntary movement. These disorders are known as hypokinetic disorders. However, a disorder leading to abnormally low output of the basal ganglia leads to relatively no inhibition, and thus excitation, of the thalamocortical projection neurons (VA and VL) which synapse onto the cortex. This situation leads to an inability to suppress unwanted movements. These disorders are known as hyperkinetic disorders.[2] Currently, reasons for abnormal increases or decreases of basal ganglia output are poorly understood. One possible factor could be the natural accumulation of iron in the basal ganglia, causing neurodegeneration due to its involvement in toxic, free-radical reactions.[3] Though motor disorders are the most common associated with the basal ganglia, recent research shows that basal ganglia disorders can lead to other dysfunctions such as obsessive compulsive disorder (OCD) and Tourette syndrome.[4]
| Basal ganglia disease | |
|---|---|
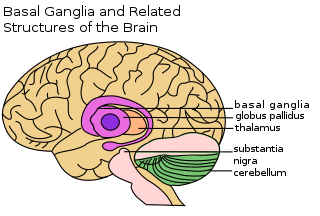 | |
| Basal ganglia | |
| Specialty | Neurology |
| Types | 8 |
Basal ganglia circuits
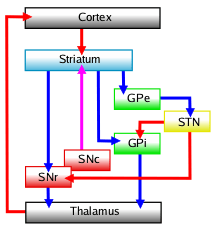
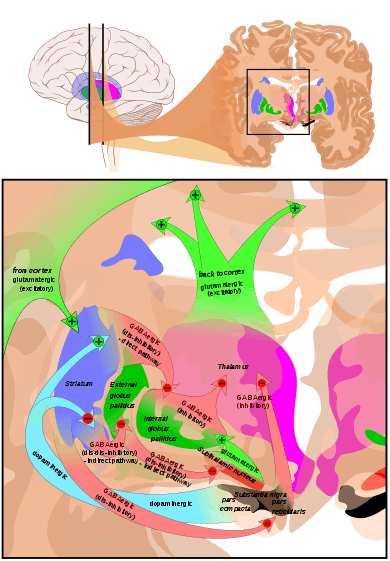
The basal ganglia is a collective group of structures in the brain. These include the striatum, (composed of the putamen and caudate nucleus), globus pallidus, substantia nigra, and the subthalamic nucleus. Along with other structures, the basal ganglia are part of a circuit that is integral to voluntary motor function.[1] It was once believed that the primary function of the basal ganglia was to integrate projections from the cerebral cortex, and project information via the thalamus to the motor cortex. New research shows that the basal ganglia can be modeled as a group of components of parallel, reentrant cortico-subcortical circuits, which originate in cortical areas, traverse the basal ganglia and terminate in specific areas in the frontal lobe.[4] These areas are thought to control not only motor function but also oculomotor, prefrontal, associative, and limbic areas.[2] Understanding these circuits has led to breakthroughs in understanding the disorders of the basal ganglia.
Direct pathway
Of all the circuits, the motor circuit is the most studied due its importance to motor disorders. The direct pathway of the motor circuit is one in which projections from the cortex travel to the putamen directly to the internal segment of the globus pallidus (GPi also known as GP-Medial) or the substantia nigra, pars reticulata (SNr) and are then directed toward the ventral anterior nucleus (VA), and the ventral lateral nucleus of the thalamus (VL) and brainstem.[2][4] Through this pathway the basal ganglia is able to initiate voluntary movements by disinhibiting thalamic neurons that drive upper motor neurons.[1] This process is regulated by dopamine secreted by the striatum onto the D1 dopamine receptor on the SNc. Dopamine excites striatal neurons in the direct pathway.[5] Proper striatal dopamine release is integral in the suppression of the basal ganglia output, which is needed for increased activity of the thalamic neurons.[2] This activity in thalamic nuclei is an integral component of voluntary movement.
Indirect pathway
The indirect pathway of the motor circuit is thought to project from the cortex, to the putamen, and to the thalamus and brainstem indirectly by passing through the external segment of the globus pallidus (GPe) then the subthalamic nucleus (STN) before looping back to the internal segment of the globus pallidus (GPi).[4] The indirect pathway is responsible for the termination of movement. The indirect pathway inhibits unwanted movements by simultaneous increase in excitatory input to other GPi and SNr neurons.[4] Similar to the direct pathway, the indirect pathway is regulated by striatal dopamine. D2 dopamine receptors inhibit transmission via the indirect pathway. D2 receptors inhibit striatal neurons in the indirect, inhibitory pathway.[5] This inhibitory effect of dopamine on the indirect pathway serves the same function as its excitatory effects in the direct pathway in that it reduces basal ganglia output, leading to the disinhibition of motor neurons.[2]
Associated disorders
Hypokinetic disorders
Hypokinetic disorders are movement disorders that are described as having reduced motor function. This is generally attributed to higher than normal basal ganglia output causing inhibition of thalamocortical motor neurons.
Parkinsonism
The muscle rigidity, tremor at rest, and slowness in initiation and execution of movement that are the cardinal motor symptoms of Parkinson's disease are attributed to a reduction in dopaminergic activity in the basal ganglia motor areas, particularly the putamen, due to gradually reduced innervation from the pars compacta of substantia nigra.[6] Other motor deficits and common non-motor features of Parkinson's such as autonomic dysfunction, cognitive impairment, and gait/balance difficulties, are thought to result from widespread progressive pathological changes commencing in the lower brain stem and ascending to the midbrain, amygdala, thalamus and ultimately the cerebral cortex.[4]
Hyperkinetic disorders
Hyperkinetic disorders are movement disorders characterized by increased uncontrollable motor function. They are caused by reduced basal ganglia output, which causes increased thalamocortical function which lead to the inability to stop unwanted movement.
Huntington's disease
Huntington’s disease is a hereditary disease that causes defects in behavior, cognition, and uncontrolled rapid, jerky movements.[1] Huntington’s disease stems from a defect that consists of an expanded CAG repeat in a gene located on chromosome 4p. Evidence shows that the basal ganglias in patients with Huntington’s Disease show a decrease in activity of the mitochondrial pathway, complex II-III. Such deficiencies are often associated with basal ganglia degeneration.[7] This degeneration of striatal neurons projecting to GPe leads to disinhibition of the indirect pathway, increased inhibition of STN, and therefore, reduced output of the basal ganglia.[2] The neuronal degeneration eventually causes death within 10 to 20 years.
Dystonia
Dystonia is a hyperkinetic movement disorder that is characterized by involuntary movement and the slowing of intentional movement. Though there are known causes of dystonia such as metabolic, vascular, and structural abnormalities, there are still patients with dystonia with no apparent cause. Dystonia can occur as a hyperkinetic disorder or as a side effect of hypokinetic disorders such as Parkinson’s disease.[8] Until recently it was thought that dystonia was likely caused by extreme lack of function of the direct pathway between the Putamen and the GPi. Again, it was thought that this dysfunction lead to a decrease in basal ganglia output to the thalamus and a resultant increased disinhibition of the thalamic projections to the premotor and motor cortex. .[9] However recent models in mice show that the dysfunction in the cerebellum may play an equal part in dystonia. .[10]
Hemiballismus
Hemiballismus is a hyperkinetic movement disorder that causes uncontrolled movement on one side of the body. It is generally caused by damage to the subthalamic nucleus (STN). Since the internal segment of the globus pallidus (GPi) is the link in the circuit between the STN and thalamic projection, destruction of localized brain cells in the GPi via a pallidotony has proven to serve as a useful treatment for Hemiballismus.[8]
Other basal ganglia diseases
The following diseases that generally involve the basal ganglia do not clearly fit into being either hypo- or hyperkinetic:
Tourette syndrome/obsessive-compulsive disorder
Tourette syndrome is a disorder that is characterized by behavioral and motor tics, OCD, and Attention-deficit hyperactivity disorder (ADHD). For this reason, it is commonly believed that pathologies involving limbic, associative, and motor circuits of the basal ganglia are likely. Since the realization that syndromes such as Tourette Syndrome and OCD are caused by dysfunction of the non-motor loops of basal ganglia circuits, new treatments for these disorders, based on treatments originally designed to treat movement disorders are being developed.[4]
Sydenham's chorea
Sydenham's chorea is a disorder characterized by rapid, uncoordinated jerking movements primarily affecting the face, hands and feet.[11] It is a result of an autoimmune response that occurs following infection by group A β-hemolytic streptococci[12] that destroys cells in the corpus striatum of the basal ganglia.[13][12][14]
Calcifications
There is physiological intracranial calcification in about 0,3-1,5% of individuals.[15] Fahr's disease is a rare,[16] genetically dominant, inherited neurological disorder characterized by abnormal deposits of calcium, primarily in the basal ganglia.
PANDAS
PANDAS is a hypothesis that there exists a subset of children with rapid onset of obsessive-compulsive disorder (OCD) or tic disorders and these symptoms are caused by group A beta-hemolytic streptococcal (GABHS) infections.[17] The proposed link between infection and these disorders is that an initial autoimmune reaction to a GABHS infection produces antibodies that interfere with basal ganglia function, causing symptom exacerbations. It has been proposed that this autoimmune response can result in a broad range of neuropsychiatric symptoms.[18][19]
Research
Gene therapy
Many disorders of the basal ganglia are due to the dysfunction of a localized area. For this reason gene therapy seems viable for neurodegenerative disorders. Gene therapy is performed by replacing diseased phenotypes with new genetic material. This process is still in the early stages but early results are promising. An example of this therapy might involve implanting cells genetically modified to express tyrosine hydroxylase which, in the body, could be converted to dopamine. Increasing dopamine levels in the basal ganglia could possibly offset the effects of the Parkinson’s Disease.[1]
Ablation
Lesionsing is the intentional destruction of neuronal cells in a particular area used for therapeutic purposes. Though this seems dangerous, vast improvements have been achieved in patients with movement disorders.[20] The exact process generally involves unilateral lesioning in the sensorimotor territory of the GPi. This process is called pallidotomy. It is believed that the success of pallidotomies in reducing the effects of movement disorders may result from the interruption of abnormal neuronal activity in the GPi. This ablation technique can be viewed as simply removing a faulty piece of a circuit. With the damaged piece of the circuit removed, the healthy area of the circuit can continue normal function.[8]
Deep brain stimulation
Deep brain stimulation involves inserting, via stereotaxic surgery, electrodes into the sensorimotor area of the brain.[1][4] These electrodes emit high-frequency stimulation to the implanted areas.[4] Bilateral implantation is necessary for symmetric results as well as the ability to reduce the intensity and duration of off-periods as well increase the duration of on-periods.[1][4] The most effective structures used for implantations for deep brain stimulation are the internal globus pallidus (GPi) and the subthalamic nucleus (STN). This is because it is safer and more effective to alter the influence of the basal ganglia on the thalamocortical nuclei than directly altering neural activity in upper motor neuron circuits.[1] Deep brain stimulation is a more complicated process than other therapies such as ablation. Evidence suggests that benefits of STN deep brain stimulation is due to the activation of efferents and the modulation of discharge patterns in the GPi that are propagated throughout the thalamocorical pathways.[4] The ability to adjust stimulation protocols lends this treatment to a variety of disorders due its ability to alter the activity of basal ganglia circuits.[1]
See also
References
- Purves, D.; Augustine, G.; Fitzpatrick, D.; Hall, W.; LaManita, A.-S.; McNamara, J.; et al. (2008). Neuroscience (4th ed.). Sunderland MA: Sinauer Associates.
- Wichmann T, DeLong MR (December 1996). "Functional and pathophysiological models of the basal ganglia". Curr. Opin. Neurobiol. 6 (6): 751–8. doi:10.1016/S0959-4388(96)80024-9. PMID 9000030.
- Curtis AR, Fey C, Morris CM, et al. (August 2001). "Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease". Nat. Genet. 28 (4): 350–4. doi:10.1038/ng571. PMID 11438811.
- DeLong MR, Wichmann T (January 2007). "Circuits and circuit disorders of the basal ganglia". Arch. Neurol. 64 (1): 20–4. doi:10.1001/archneur.64.1.20. PMID 17210805.
- Nambu A (December 2008). "Seven problems on the basal ganglia". Curr. Opin. Neurobiol. 18 (6): 595–604. doi:10.1016/j.conb.2008.11.001. PMID 19081243.
- Heinz Steiner; Kuei Y. Tseng (4 January 2010). Handbook of Basal Ganglia Structure and Function. Academic Press. p. 663. ISBN 978-0-12-374767-9. Retrieved 20 April 2012.
- Beal MF (August 1998). "Mitochondrial dysfunction in neurodegenerative diseases". Biochim. Biophys. Acta. 1366 (1–2): 211–23. doi:10.1016/s0005-2728(98)00114-5. PMID 9714810.
- Vitek JL, Chockkan V, Zhang JY, et al. (July 1999). "Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus". Ann. Neurol. 46 (1): 22–35. doi:10.1002/1531-8249(199907)46:1<22::AID-ANA6>3.0.CO;2-Z. PMID 10401777.
- Janavs JL, Aminoff MJ (October 1998). "Dystonia and chorea in acquired systemic disorders". J. Neurol. Neurosurg. Psychiatry. 65 (4): 436–45. doi:10.1136/jnnp.65.4.436. PMC 2170280. PMID 9771763.
- Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA (September 2008). "The basal ganglia and cerebellum interact in the expression of dystonic movement". Brain. 131 (Pt 9): 2499–509. doi:10.1093/brain/awn168. PMC 2724906. PMID 18669484.
- "Sydenham Chorea Information Page" Archived 2010-07-22 at the Wayback Machine Saint Vitus Dance, Rheumatic Encephalitis from the National Institute of Neurological Disorders and Stroke. Accessed April 26, 2008
- Sydenham's Chorea Symptoms.Accessed September 24, 2009. Archived April 18, 2008, at the Wayback Machine
- Swedo SE, Leonard HL, Shapiro MB (1993). "Sydenham's Chorea:Physical and Psychological Symptoms of St Vitus Dance". Pediatrics. 91 (4): 706–713.
- Faustino PC, Terreri MT, Rocha AJ, et al. (2003). "Clinical, laboratory, psychiatric and magnetic resonance findings in patients with sydenham chorea". Neuroradiology. 45 (7): 456–462. doi:10.1007/s00234-003-0999-8. PMID 12811441.
- Verulashvili IV, Glonti LSh, Miminoshvili DK, Maniia MN, Mdivani KS (2006). "[Basal ganglia calcification: clinical manifestations and diagnostic evaluation]". Georgian Med News (in Russian) (140): 39–43. PMID 17179586.
- "Archived copy". Archived from the original on 2009-05-11. Retrieved 2009-06-13.CS1 maint: archived copy as title (link)
- Moretti G, Pasquini M, Mandarelli G, Tarsitani L, Biondi M (2008). "What every psychiatrist should know about PANDAS: a review". Clin Pract Epidemiol Ment Health. 4 (1): 13. doi:10.1186/1745-0179-4-13. PMC 2413218. PMID 18495013.
- de Oliveira SK, Pelajo CF (March 2010). "Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infection (PANDAS): a Controversial Diagnosis". Curr Infect Dis Rep. 12 (2): 103–9. doi:10.1007/s11908-010-0082-7. PMID 21308506.
- Boileau B (2011). "A review of obsessive-compulsive disorder in children and adolescents". Dialogues Clin Neurosci. 13 (4): 401–11. PMC 3263388. PMID 22275846.
- Baron MS, Vitek JL, Bakay RA, et al. (September 1996). "Treatment of advanced Parkinson's disease by posterior GPi pallidotomy: 1-year results of a pilot study". Ann. Neurol. 40 (3): 355–66. doi:10.1002/ana.410400305. PMID 8797525.