Brinzolamide
Brinzolamide (trade names Azopt, Alcon Laboratories, Befardin,[1] Fardi Medicals,[2] ) is a carbonic anhydrase inhibitor used to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
 | |
| Clinical data | |
|---|---|
| Trade names | Azopt, Befardin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601233 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Ophthalmic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Absorbed systemically, but below detectable levels (less than 10 ng/mL) |
| Protein binding | ~60% |
| Elimination half-life | 111 days |
| Excretion | Renal (60%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.376 |
| Chemical and physical data | |
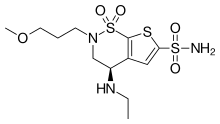
| Formula | C12H21N3O5S3 |
| Molar mass | 383.51 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Chemistry
Brinzolamide is a carbonic anhydrase inhibitor (specifically, carbonic anhydrase II). Carbonic anhydrase is found primarily in erythrocytes (but also in other tissues including the eye). It exists as a number of isoenzymes, the most active of which is carbonic anhydrase II (CA-II).
Indications
Use for the treatment of open-angle glaucoma and raised intraocular pressure due to either excess aqueous humor production or inadequate drainage of the humor via the trabecular meshwork.
Pharmacodynamics
Inhibition of carbonic anhydrase in the ciliary processes of the eye decreases aqueous humor secretion and thus lowers the intraocular pressure in the anterior chamber, presumably by reducing the rate of formation of bicarbonate ions with subsequent reduction in sodium and fluid transport; this may alleviate the effects of open-angle glaucoma.
Pharmacokinetics
Absorption
The recommended frequency for topical application is two times per day. Following ocular instillation, the suspension is systemically absorbed to some degree; however the plasma concentrations are low and generally below the limits of detection (less than 10 ng/mL) due to extensive binding by tissues and erythrocytes. Oral administration is less-favored due to variable absorption from the stomach mucosa and an increased side-effect profile versus ophthalmic administration.
Distribution
The compound is fairly well protein-bound (60%), but adheres extensively to the carbonic anhydrase-containing erythrocytes. Due to the abundance of readily-bound erythrocytes and minimal known metabolism, Brinzolamide's whole blood half-life is very long (111 days).
Metabolism
While definitive sites of metabolism have not been firmly established, there are several metabolites worthy of note. N-Desethylbrinzolamide is an active metabolite of the parent compound, and thus exhibits carbonic anhydrase inhibitory activity (largely carbonic anhydrase-I, when in the presence of Brinzolamide) and also accumulates in the erythrocytes. However, Brinzolamide's other known metabolites (N-Desmethoxypropylbrinzolamide and O-Desmethylbrinzolamide) either have no activity or their activity is currently unknown.
Excretion
Brinzolamide is excreted primarily unchanged (60%) in the urine, although the renal clearance rate has not been definitively determined. N-Desethylbrinzolamide is also found in the urine along with lower concentrations of the inactive metabolites, N-Desmethoxypropylbrinzolamide and O-Desmethylbrinzolamide; exact levels have not been definitively determined.
Cautions
Side effects
- Common, but mild: blurred vision; bitter, sour, or unusual taste; itching, pain, watering, or dryness of the eyes; feeling that something is in the eye; headache; runny nose
- Rare, but serious: fast or irregular heartbeat; fainting; skin rash, hives, or itching; severe eye irritation, redness, or swelling; swelling in the face, lips, or throat; wheezing or trouble breathing
Precautions
- Hypersensitivity to other sulfonamides
- Acute angle-closure glaucoma
- Concomitant administration of oral carbonic anhydrase inhibitors
- Moderate-to-severe chronic kidney disease or liver disease
Combination with timolol
The combination of brinzolamide with timolol is marketed under the trade name Azarga. This combination may be more effective than either medication alone.[3]
References
- "Archived copy". Archived from the original on 2015-07-06. Retrieved 2015-07-04.CS1 maint: archived copy as title (link)
- "Archived copy" (PDF). Archived from the original (PDF) on 2015-07-05. Retrieved 2015-07-04.CS1 maint: archived copy as title (link)
- Croxtall JD, Scott, LJ. Archived 2011-10-08 at the Wayback Machine.Drugs&Aging 2009;26(5):437-446. doi:10.2165/00002512-200926050-00007.
- Ermis S, Ozturk F, Inan U (2005). "Comparing the effects of travoprost and brinzolamide on intraocular pressure after phacoemulsification". Eye. 19 (3): 303–7. doi:10.1038/sj.eye.6701470. PMID 15258611.
- Iester M, Altieri M, Michelson G, Vittone P, Traverso C, Calabria G (2004). "Retinal peripapillary blood flow before and after topical brinzolamide". Ophthalmologica. 218 (6): 390–6. doi:10.1159/000080942. PMID 15564757.
- Kaup M, Plange N, Niegel M, Remky A, Arend O (2004). "Effects of brinzolamide on ocular haemodynamics in healthy volunteers". Br J Ophthalmol. 88 (2): 257–62. doi:10.1136/bjo.2003.021485. PMC 1771998. PMID 14736787.
- March WF, Ochsner KI (2000). "The long-term safety and efficacy of brinzolamide 1.0% (azopt) in patients with primary open-angle glaucoma or ocular hypertension". Am J Ophthalmol. 129 (2): 136–143. doi:10.1016/S0002-9394(99)00343-8. PMID 10682964.
- Product Information: Azopt(R), brinzolamide. Alcon Laboratories, Inc, Fort Worth, TX, 1998b.
- Sall KN, Greff LJ, Johnson-Pratt LR, et al.: Dorzolamide/timolol combination versus concomitant administration of brimonidine and timolol: six-month comparison of efficacy and tolerability. Ophthalmology 2003; 110:615-624.