Amyloid-related imaging abnormalities
Amyloid-related imaging abnormalities (ARIA) are abnormal differences seen in neuroimaging of Alzheimer's Disease patients, associated with amyloid-modifying therapies, particularly human monoclonal antibodies such as aducanumab.[1] There are two types of ARIA - ARIA-E and ARIA-H. The phenomenon was first seen in trials of bapineuzumab.[2]
| Amyloid-related imaging abnormalities | |
|---|---|
| Other names | ARIA |
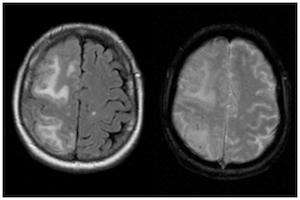 | |
| Two MRI scans demonstrating the difference between ARIA-E (left) and ARIA-H in the parietal region (right) | |
| Specialty | Radiology, neurology |
ARIA-E
ARIA-E refers to cerebral edema, involving the breakdown of the tight endothelial junctions of the blood-brain barrier and subsequent accumulation of fluid.[3] In a double-blind trial of the humanised monoclonal antibody solanezumab (n = 2042), sixteen patients (11 taking the drug, 5 taking a placebo), or 0.78% developed ARIA-E. A further 7 patients developed ARIA-E during an open-label extension of the trial.[4]
The effect of ARIA-E depends on the severity and location of the oedema. Symptoms may include headache, changes in mental state, confusion, vomiting, nausea, tremor and gait disturbances.[4]
ARIA-H
ARIA-H refers to cerebral microhaemorrhages (mH), small haemorrhages on the brain,[5] often accompanied by hemosiderosis.[1] mH is usually seen as small, round and low intensity lesions and are small haemosiderin deposits. Some studies define mH as being less than or equal to 10mm, while others define the cut-off as ≤ 5mm.[1] The prevalence of mH in healthy elderly people is approximately 6%, but this value increases to between 50% and 80% in elderly people with cerebrovascular disease.[6]
References
- Sperling, Reisa A.; Jack, Clifford R.; Black, Sandra E.; Frosch, Matthew P.; Greenberg, Steven M.; Hyman, Bradley T.; Scheltens, Philip; Carrillo, Maria C.; Thies, William (2016-12-12). "Amyloid Related Imaging Abnormalities (ARIA) in Amyloid Modifying Therapeutic Trials: Recommendations from the Alzheimer's Association Research Roundtable Workgroup". Alzheimer's & Dementia : The Journal of the Alzheimer's Association. 7 (4): 367–385. doi:10.1016/j.jalz.2011.05.2351. ISSN 1552-5260. PMC 3693547. PMID 21784348.
- DiFrancesco, Jacopo C.; Longoni, Martina; Piazza, Fabrizio (2015-09-25). "Anti-Aβ Autoantibodies in Amyloid Related Imaging Abnormalities (ARIA): Candidate Biomarker for Immunotherapy in Alzheimer's Disease and Cerebral Amyloid Angiopathy". Frontiers in Neurology. 6: 207. doi:10.3389/fneur.2015.00207. ISSN 1664-2295. PMC 4585101. PMID 26441825.
- "Paris: Renamed ARIA, Vasogenic Edema Common to Anti-Amyloid Therapy | ALZFORUM". www.alzforum.org. Retrieved 2016-12-11.
- Carlson, Christopher; Siemers, Eric; Hake, Ann; Case, Michael; Hayduk, Roza; Suhy, Joyce; Oh, Joonmi; Barakos, Jerome (2016-03-02). "Amyloid-related imaging abnormalities from trials of solanezumab for Alzheimer's disease". Alzheimer's & Dementia : Diagnosis, Assessment & Disease Monitoring. 2: 75–85. doi:10.1016/j.dadm.2016.02.004. ISSN 2352-8729. PMC 4879647. PMID 27239538.
- Gaillard, Frank. "Cerebral microhaemorrhage | Radiology Reference Article | Radiopaedia.org". radiopaedia.org. Retrieved 2016-12-11.
- Fiehler, Jens (2006-08-01). "Cerebral microbleeds: old leaks and new haemorrhages". International Journal of Stroke. 1 (3): 122–130. doi:10.1111/j.1747-4949.2006.00042.x. ISSN 1747-4949. PMID 18706032.