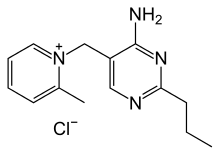
Amprolium
Amprolium is the organic compound sold as a coccidiostat used in poultry. It has many International Nonproprietary Names.[1]
 | |
| Names | |
|---|---|
| IUPAC name
5-[(2-methylpyridin-1-ium-1-yl)methyl]-2-propyl-pyrimidin-4-amine chloride | |
| Other names
1-[(4-Amino-2-propyl-5-pyrimidinyl)methyl]-2-picolinium chloride, Amprovine, Amprolium, Amprol, Anticoccid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.054 |
| EC Number |
|
| MeSH | Amprolium |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C14H19N4+ · Cl− |
| Molar mass | 278.780 g·mol−1 |
| Pharmacology | |
| QP51AX09 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses in poultry farming
The drug is a thiamine analogue and blocks the thiamine transporter of Eimeria species. By blocking thiamine uptake it prevents carbohydrate synthesis.
Despite only moderate efficacy it is well favoured due to few resistance issues and is commonly used in the United States in conjunction with sulfonamides prophylactically in chickens and cattle as a coccidiostat.
Coccidia are protozoans that can wreak havoc in a flock of poultry by an infection known as coccidiosis. Agents that control this disease–coccidiostats–are in view of the world's heavy dependence on poultry as a source of protein, of great economical significance.
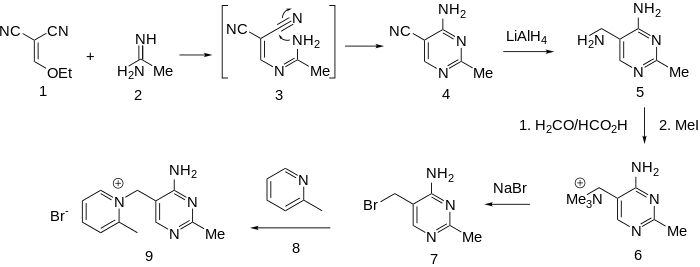
Production
Condensation of ethoxymethylenemalononitrile (1) with acetamidine (2) affords the substituted pyrimidine (4). The rxn may well involve conjugate addition of the amidine nitrogen to the malononitrile followed by loss of ethoxide (3); addition of the remaining amidine nitrogen to one of the nitriles will then lead to the pyrimidine (4). Reduction of the nitrile gives the corresponding aminomethyl compound (5). Exhaustive methylation of the amine followed by displacement of the activated quaternary nitrogen by bromide ion affords the key intermediate (7). Displacement of the halogen by α-picoline gives amprolium.
References
- Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
- Grewe, R. Z. Physiol. Chem. (1936).
- "Archived copy". Archived from the original on 2015-04-02. Retrieved 2015-03-30.CS1 maint: archived copy as title (link)