Amoeboid movement
Amoeboid movement is the most common mode of locomotion in eukaryotic cells.[1] It is a crawling-like type of movement accomplished by protrusion of cytoplasm of the cell involving the formation of pseudopodia ("false-feet") and posterior uropods. One or more pseudopodia may be produced at a time depending on the organism, but all amoeboid movement is characterized by the movement of organisms with an amorphous form that possess no set motility structures. Movement occurs when the cytoplasm slides and forms a pseudopodium in front to pull the cell forward. This type of movement has been linked to changes in action potential, though the exact mechanism is still unknown. Some examples of organisms that exhibit this type of locomotion are the amoeboids, slime molds and some protozoans such as Naegleria gruberi,[2] as well as some cells in humans such as leukocytes. Sarcomas, or cancers arising from connective tissue cells, are particularly adept at amoeboid movement, thus leading to their high rate of metastasis.
While several hypotheses have been proposed to explain the mechanism of amoeboid movement, the exact mechanism is still unknown.[3]
Molecular mechanism of cell motion
Sol-gel theory
The protoplasm of an amoeba is made up of an outer layer termed the ectoplasm which surrounds an inner portion called the endoplasm. The ectoplasm consists of a gelatinous semisolid called plasma gel whereas the endoplasm is made up of a less viscous fluid called plasma sol. The ectoplasm owes its highly viscous state, in part, to the cross-linking actomyosin complex. Locomotion of an amoeba is thought to occur due to the sol-gel conversion of the protoplasm within its cell. 'Sol-gel conversion describes the contraction and relaxation events which are enforced by osmotic pressure and other ionic charges.'[4]
For example, when an amoeba moves, it extends a gelatinous, cytosolic pseudopodium, which then results in the more fluid cytosol (plasma sol) flowing after the gelatinous portion (plasma gel) where it congeals at the end of the pseudopodium. This results in the extension of this appendage. On the opposite (posterior) end of the cell, plasma gel is then converted to plasma sol and streamed toward the advancing pseudopodium. As long as the cell has a way to grapple the substratum, repetition of this process guides the cell forward. Inside the amoeba, there are proteins that can be activated to convert the gel into the more liquid sol state.
Cytoplasm consist largely of actin and actin is regulated by actin binding proteins. Actin binding proteins are in turn regulated by calcium ions; hence, calcium ions are very important in the sol-gel conversion process.[1][4]
Amoeboid movement modalities
Actin-driven motility
Based on some mathematical models, recent studies hypothesize a novel biological model for collective biomechanical and molecular mechanisms of cellular motion.[5] It is proposed that microdomains weave the texture of cytoskeleton and their interactions mark the location for formation of new adhesion sites. According to this model, microdomain signaling dynamics organize the cytoskeleton and its interaction with the substratum. As microdomains trigger and maintain active polymerization of actin filaments, their propagation and zigzagging motion on the membrane generate a highly interlinked network of curved or linear filaments oriented at a wide spectrum of angles to the cell boundary. It has also been proposed that microdomain interaction marks the formation of new focal adhesion sites at the cell periphery. The interaction of myosin with the actin network then generates membrane retraction/ruffling, retrograde flow, and contractile forces for forward motion. Finally, continuous application of stress on the old focal adhesion sites could result in the calcium-induced activation of calpain, and consequently the detachment of focal adhesions which completes the cycle.
In addition to actin polymerization, microtubules may also play an important role in cell migration where the formation of lamellipodia is involved. One experiment showed that although microtubules are not required for actin polymerization to create lamellipodial extensions, they are needed in order to afford cellular movement.[6]
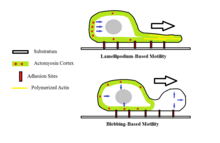
Bleb-driven motility
Another such proposed mechanism, the 'bleb-driven amoeboid locomotion' mechanism, suggests that the cell cortex actomyosin contracts to increase hydrostatic pressure inside the cell. Blebbing occurs in amoeboid cells when there is a roughly spherical protrusion in the cell membrane characterized by detachment from the actomyosin cortex. This mode of amoeboid movement requires that myosin II play a role in generating the hydrostatic pressure that causes the bleb to extend.[7] This is different from actin-driven locomotion where the protrusion created is by the actin polymerizing while remaining attached to the actomyosin cortex and physically pushing against the cell's barrier. During the bleb-driven amoeboid movement, the cytoplasmic sol-gel state is regulated.[1]
Blebbing can also be a sign of when a cell is undergoing apoptosis.[8]
It has also been observed that the blebs formed by motile cells undergo a roughly uniform life cycle that lasts approximately one minute. This includes a phase involving the initial outward expansion where the membrane breaks away from the membranous cytoskeleton. This is then followed by a short static phase where the hydrostatic pressure that has built up is just enough to maintain the size of the bleb. Following this is the last phase characterized by the bleb slowly retracting and the membrane being reintroduced to the cytoskeleton infrastructure.[9]
Cells may undergo fast transitions between blebbing and lamellipodium-based motility as a means of migration. However, the rate at which these transitions are made is still unknown. Tumor cells may also exhibit rapid transitions between amoeboid motility and mesenchymal motility, another form of cellular movement.[10]
See also
- Cytoplasmic streaming
- Category:Cell Movement
- Pseudopodium
- Amoeba
References
- Nishigami, Yukinori; Ichikawa, Masatoshi; Kazama, Toshiya; Kobayashi, Ryo; Shimmen, Teruo; Yoshikawa, Kenichi; Sonobe, Seiji; Kabla, Alexandre J. (5 August 2013). "Reconstruction of Active Regular Motion in Amoeba Extract: Dynamic Cooperation between Sol and Gel States". PLoS ONE. 8 (8): e70317. doi:10.1371/journal.pone.0070317. PMC 3734023. PMID 23940560.
- Preston, TM; Cooper, LG; King, CA (Jul–Aug 1990). "Amoeboid locomotion of Naegleria gruberi: the effects of cytochalasin B on cell-substratum interactions and motile behavior". The Journal of Protozoology. 37 (4): 6S–11S. doi:10.1111/j.1550-7408.1990.tb01139.x. PMID 2258833.
- R D Allen, and N S Allen. "Cytoplasmic Streaming in Amoeboid Movement" http://www.annualreviews.org/doi/abs/10.1146/annurev.bb.07.060178.002345 Allen, R. D.; Allen, N. S. (1978). "Cytoplasmic Streaming in Amoeboid Movement". Annual Review of Biophysics and Bioengineering. 7: 469–495. doi:10.1146/annurev.bb.07.060178.002345. PMID 352246.
- Rastogi, S.C. (2010). Cell and molecular biology (3rd ed.). New Delhi: New Age International. p. 461. ISBN 9788122430790. Retrieved 29 October 2014.
- Coskun, Hasan; Coskun, Huseyin. (March 2011). "Cell physician: reading cell motion. A mathematical diagnostic technique through analysis of single cell motion". Bull Math Biol. 73 (3): 658–82. doi:10.1007/s11538-010-9580-x. PMID 20878250.
- Ballestrem, Christoph; Wehrle-Haller, Bernhard; Hinz, Boris; Imhof, Beat A. (2000-09-01). "Actin-dependent Lamellipodia Formation and Microtubule-dependent Tail Retraction Control-directed Cell Migration". Molecular Biology of the Cell. 11 (9): 2999–3012. doi:10.1091/mbc.11.9.2999. ISSN 1059-1524. PMC 14971. PMID 10982396.
- Yoshida, Kunito; Soldati, Thierry (2006-09-15). "Dissection of amoeboid movement into two mechanically distinct modes". Journal of Cell Science. 119 (18): 3833–3844. doi:10.1242/jcs.03152. ISSN 0021-9533. PMID 16926192.
- Coleman, Mathew L.; Sahai, Erik A.; Yeo, Margaret; Bosch, Marta; Dewar, Ann; Olson, Michael F. (2001). "Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I". Nature Cell Biology. 3 (4): 339–345. doi:10.1038/35070009. ISSN 1476-4679. PMID 11283606.
- Fackler, Oliver T.; Grosse, Robert (2008-06-16). "Cell motility through plasma membrane blebbing". The Journal of Cell Biology. 181 (6): 879–884. doi:10.1083/jcb.200802081. ISSN 0021-9525. PMC 2426937. PMID 18541702.
- Bergert, Martin; Chandradoss, Stanley D.; Desai, Ravi A.; Paluch, Ewa (2012-09-04). "Cell mechanics control rapid transitions between blebs and lamellipodia during migration". Proceedings of the National Academy of Sciences. 109 (36): 14434–14439. doi:10.1073/pnas.1207968109. ISSN 0027-8424. PMC 3437886. PMID 22786929.
- Van Haastert, Peter J. M.; Hotchin, Neil A. (8 November 2011). Hotchin, Neil A (ed.). "Amoeboid Cells Use Protrusions for Walking, Gliding and Swimming". PLoS ONE. 6 (11): e27532. doi:10.1371/journal.pone.0027532. PMC 3212573. PMID 22096590.
- Bae, A. J.; Bodenschatz, E. (4 October 2010). "On the swimming of Dictyostelium amoebae". Proceedings of the National Academy of Sciences. 107 (44): E165–E166. doi:10.1073/pnas.1011900107. PMC 2973909. PMID 20921382.