Amatoxin
Amatoxin is the collective name of a subgroup of at least eight related toxic compounds found in several genera of poisonous mushrooms, most notably the death cap (Amanita phalloides) and several other members of the genus Amanita, as well as some Conocybe, Galerina and Lepiota mushroom species. Amatoxins are lethal in even small doses, as little as half a mushroom. Unlike many ingested poisons, they are not destroyed by heat (except at a temperature that would make it inedible and non-nutritious), so cooking the poisonous mushrooms does not diminish their lethality.
Structure
The compounds have a similar structure, that of eight amino-acid residues arranged in a conserved macrobicyclic motif (an overall pentacyclic structure when counting the rings inherent in the proline and tryptophan-derived residues); they were isolated in 1941 by Heinrich O. Wieland and Rudolf Hallermayer.[1] All amatoxins are oligopeptides that are synthesized as 35-amino-acid proproteins, from which the final eight amino acids are cleaved by a prolyl oligopeptidase.[2] The schematic amino acid sequence of amatoxins is Ile-Trp-Gly-Ile-Gly-Cys-Asn-Pro with cross-linking between Trp and Cys via the sulfoxide (S=O) moiety and hydroxylation in variants of the molecule.
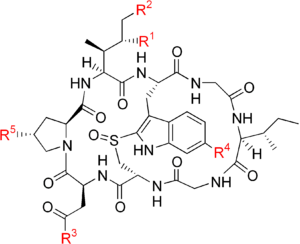
There are currently ten known amatoxins:[3]
| Name | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| α-Amanitin | OH | OH | NH2 | OH | OH |
| β-Amanitin | OH | OH | OH | OH | OH |
| γ-Amanitin | OH | H | NH2 | OH | OH |
| ε-Amanitin | OH | H | OH | OH | OH |
| Amanullin | H | H | NH2 | OH | OH |
| Amanullinic acid | H | H | OH | OH | OH |
| Amaninamide | OH | OH | NH2 | H | OH |
| Amanin | OH | OH | OH | H | OH |
| Proamanullin | H | H | NH2 | OH | H |
δ-Amanitin has been reported, but its chemical structure has not been determined.
Mechanism
Amatoxins are potent and selective inhibitors of RNA polymerase II, a vital enzyme in the synthesis of messenger RNA (mRNA), microRNA, and small nuclear RNA (snRNA). Without mRNA, which is the template for protein synthesis, cell metabolism stops and lysis ensues.[4] The RNA polymerase of Amanita phalloides is insensitive to the effects of amatoxins; thus, the mushroom does not poison itself.[5]
Amatoxins are able to travel through the bloodstream to reach the organs in the body. While these compounds can damage many organs, damage to the liver and heart result in fatalities. At the molecular level, amatoxins cause damage to cells of these organs by causing perforations in the plasma membranes resulting in misplaced organelles that are normally in the cytoplasm to be found in the extracellular matrix.[6] beta-Amanitin is also an inhibitor of eukaryotic RNA polymerase II and RNA polymerase III, and as a result, mammalian protein synthesis. It has not been found to inhibit RNA polymerase I or bacterial RNA polymerase.[7] Because it inactivates the RNA polymerases, the liver is unable to repair the damage that beta-amanitin causes and the cells of the liver disintegrate and the liver dissolves.[8]

Alpha-amanitin (α-Amanitin) primarily affects the bridge helix of the RNA pol II complex, a highly conserved domain 35 amino acids long. At the N-terminus and the C-terminus of this region there are hinge structures that undergo significant conformational changes throughout the nucleotide addition cycle, and are essential for its progression.[10] One of the many roles of the bridge helix is facilitating the translocation of DNA.[11] Alpha-amanitin binds to the bridge helix of the RNA Pol II complex and it also binds to part of the complex that is adjacent to the bridge helix, while it is in one specific conformation. This binding locks the bridge helix into place, dramatically slowing its movement in translocating the DNA.[9] The rate of pol II translocation of DNA is reduced from several thousand to a few nucleotides per minute.[12][13]
Symptoms of exposure
Upon exposure to amatoxins, the liver is the principal organ affected as it is the organ which is first encountered after absorption in the gastrointestinal tract. There is no evidence that amatoxins are absorbed through skin. One study done on mice shows that alpha-Amanitin is not absorbed through skin and therefore can not have any toxic effects.[14] More specifically, exposure to amatoxins may cause irritation of the respiratory tract, headache, dizziness, nausea, shortness of breath, coughing, insomnia, diarrhea, gastrointestinal disturbances, back pain, urinary frequency, liver and kidney damage, or death if ingested or inhaled. For example, if β-amanitin comes in contact with skin, it may cause irritation, burns, redness, severe pain, and could be absorbed through the skin, causing similar effects to exposure via inhalation and ingestion. Contact with the eyes may result in irritation, corneal burns, and eye damage. Persons with pre-existing skin, eye, or central nervous systems disorders, impaired liver, kidney, or pulmonary function may be more susceptible to the effects of this substance.[15]
Amatoxin poisoning shows a biphasic clinical pattern. An initial (12–24 hours) period of acute symptoms is followed by a period of relative wellness that lasts for 12–24 hours. After this period, hepatic and renal failure supervene with death typically occurring from day 2 onwards. [16]
The estimated minimum lethal dose is 0.1 mg/kg or 7 mg of toxin in adults. Their swift intestinal absorption coupled with their thermostability leads to rapid development of toxic effects in a relatively short period of time. The most severe effects are toxic hepatitis with centrolobular necrosis and hepatic steatosis, as well as acute tubulointerstitial nephropathy, which altogether induce severe liver failure and kidney failure.
Treatment
There are many anecdotal and partially-studied treatments in use worldwide. One study in mice showed null results for all studied treatments. Treatments showing no discernable value included N-acetylcysteine, benzylpenicillin, cimetidine, thioctic acid, and silybin.[17]
Treatment involves high-dose penicillin as well as supportive care in cases of hepatic and renal injury. Silibinin, a product found in milk thistle, is a potential antidote to amatoxin poisoning, although more data needs to be collected. Cautious attention is given to maintaining hemodynamic stability, although if hepatorenal syndrome has developed the prognosis is guarded at best.[18]
Detection
Presence of amatoxins in mushroom samples may be detected by the Meixner test (also known as the Wieland test). The amatoxins may be quantitated in plasma or urine using chromatographic techniques to confirm a diagnosis of poisoning in hospitalized patients and in postmortem tissues to aid in a medicolegal investigation of a suspected fatal overdosage.[19]
Mushroom species
Amatoxin-containing mushroom species from the genera Amanita, Galerina and Lepiota.[20]
| Amanita species | Galerina species | Lepiota species |
|---|---|---|
| Amanita phalloides | Galerina badipes | Lepiota brunneoincarnata |
| Amanita bisporigera | Galerina beinrothii | Lepiota brunneolilacea |
| Amanita decipiens | Galerina fasciculate | Lepiota castanea |
| Amanita hygroscopica | Galerina helvoliceps | Lepiota clypeolaria |
| Amanita ocreata | Galerina marginata | Lepiota clypeolarioides |
| Amanita suballiacea | Galerina sulciceps | Lepiota felina |
| Amanita tenuifolia | Galerina unicolor | Lepiota fulvella |
| Amanita verna | Galerina venenata | Lepiota fuscovinacea |
| Amanita virosa | Lepiota griseovirens | |
| Lepiota heimii | ||
| Lepiota helveoloides | ||
| Lepiota kuehneri | ||
| Lepiota langei | ||
| Lepiota lilacea | ||
| Lepiota locanensis | ||
| Lepiota ochraceofulva | ||
| Lepiota pseudohelveola | ||
| Lepiota pseudolilacea | ||
| Lepiota rufescens | ||
| Lepiota subincarnata | ||
| Lepiota xanthophylla |
See also
- Phallotoxins, a closely related class of mycotoxins
References
- Litten, W. (March 1975). "The most poisonous mushrooms". Scientific American. 232 (3): 90–101. Bibcode:1975SciAm.232c..90L. doi:10.1038/scientificamerican0375-90. PMID 1114308.
- H. E. Hallen; H. Luo; J. S. Scott-Craig; J. D. Walton (2007). "Gene family encoding the major toxins of lethal Amanita mushrooms". Proceedings of the National Academy of Sciences of the United States of America. 104 (48): 19097–19101. Bibcode:2007PNAS..10419097H. doi:10.1073/pnas.0707340104. PMC 2141914. PMID 18025465.
- K. Baumann; K. Muenter; H. Faulstich (1993). "Identification of structural features involved in binding of α-amanitin to a monoclonal antibody". Biochemistry. 32 (15): 4043–4050. doi:10.1021/bi00066a027. PMID 8471612.
- Karlson-Stiber C, Persson H (2003). "Cytotoxic fungi - an overview". Toxicon. 42 (4): 339–49. doi:10.1016/S0041-0101(03)00238-1. PMID 14505933.
- Horgen, Paul A.; Vaisius, Allan C.; Ammirati, Joseph F. (1978). "The insensitivity of mushroom nuclear RNA polymerase activity to inhibition by amatoxins". Archives of Microbiology. 118 (3): 317–9. doi:10.1007/BF00429124. PMID 567964.
- J. Meldolesi; G. Pelosi; A. Brunelli; E. Genovese (1966). "Electron Microscopic Studies on the Effects of Amanitin in Mice: Liver and Heart Lesions". Virchows Archiv A. 342 (3): 221–235. doi:10.1007/bf00960591. PMID 5301504.
- "β-Amanitin from Amanita phalloides", Retrieved on 12 March 2013.
- "Polypeptide Toxins in Amanita Mushrooms", “Cornell University”, Retrieved on 12 March 2013.
- Bushnell, D. A.; Cramer, P; Kornberg, RD (Feb 2002). "Structural basis of transcription: alpha-amanitin-RNA polymerase II cocrystal at 2.8 A resolution". Proc Natl Acad Sci USA. 99 (3): 1218–1222. Bibcode:2002PNAS...99.1218B. doi:10.1073/pnas.251664698. PMC 122170. PMID 11805306.
- Weinzierl, R.O.J. (Sep 2011). "The Bridge Helix of RNA Polymerase Acts as a Central Nanomechanical Switchboard for Coordinating Catalysis and Substrate Movement". Archaea. 2011: 608385. doi:10.1155/2011/608385. PMC 3270539. PMID 22312317.
- Hein, P.P.; Landick, R. (2010). "The bridge helix coordinates the movements of modules in RNA polymerase". BMC Biology. 8: 141. doi:10.1186/1741-7007-8-141. PMC 2993669. PMID 21114873.
- Chafin, D. R.; Guo, H.; Price, D. H. (1995). "Action of alpha-Amanitin during Pyrophosphorolysis and Elongation by RNA Polymerase II". J. Biol. Chem. 270 (32): 19114–19119. doi:10.1074/jbc.270.32.19114. PMID 7642577.
- Rudd, M. D.; Luse, D. S. (1996). "Amanitin Greatly Reduces the Rate of Transcription by RNA Polymerase II Ternary Complexes but Fails to Inhibit Some Transcript Cleavage Modes". J. Biol. Chem. 271 (35): 21549–21558. doi:10.1074/jbc.271.35.21549. PMID 8702941.
- Kaya, E., Surmen, M. G., Yaykasli, K. O., Karahan, S., Oktay, M., Turan, H., ... & Erdem, H. (2014). Dermal absorption and toxicity of alpha amanitin in mice. Cutaneous and ocular toxicology, 33(2), 154-160.
- "Material Safety Data Sheet for beta Amanitin", Retrieved on 12 March 2013.
- "Principles of and Practice of Infectious Disease" , Retrieved on 17 March 2017.
- Tong, TC; Hernandez, M; Richardson, WH; et al. (Sep 2007). "Comparative treatment of alpha-amanitin poisoning with N-acetylcysteine, benzylpenicillin, cimetidine, thioctic acid, and silybin in a murine model". Ann Emerg Med. 50 (3): 282–8. doi:10.1016/j.annemergmed.2006.12.015. PMID 17559970.
- Piqueras J. (1989). "Hepatotoxic mushroom poisoning: diagnosis and management". Mycopathologia. 105 (2): 99–110. doi:10.1007/bf00444032. PMID 2664527.
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 52–54.
- Enjalbert, Françoise; Rapior, Sylvie; Nouguier-Soulé, Janine; Guillon, Sophie; Amouroux, Noël; Cabot, Claudine (26 November 2002). "Treatment of Amatoxin Poisoning: 20-Year Retrospective Analysis". Journal of Toxicology: Clinical Toxicology. 40 (6): 715–757. doi:10.1081/CLT-120014646. PMID 12475187.
