Alpha-thalassemia
Alpha-thalassemia (α-thalassemia, α-thalassaemia) is a form of thalassemia involving the genes HBA1[5] and HBA2.[6] Thalassemias are a group of inherited blood conditions which result in the impaired production of hemoglobin, the molecule that carries oxygen in the blood.[7] Normal hemoglobin consists of two alpha chains and two beta chains; in alpha-thalassemia, there is a quantitative decrease in the amount of alpha chains, resulting in fewer normal hemoglobin molecules. Furthermore, alpha-thalassemia leads to the production of unstable beta globin molecules which cause increased red blood cell destruction. The degree of impairment is based on which clinical phenotype is present (how many genes are affected).[3]
| Alpha-thalassemia | |
|---|---|
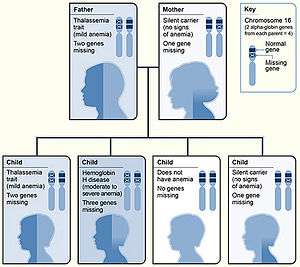 | |
| Alpha-thalassemia inheritance pattern | |
| Specialty | Hematology |
| Symptoms | Jaundice, Fatigue[1] |
| Causes | Deletions of chromosome 16p.[2] |
| Diagnostic method | Haemoglobin electrophoresis[3] |
| Treatment | Blood transfusion, possible splenectomy[1][4] |
Signs and symptoms
The presentation of individuals with alpha-thalassemia consists of:
| Common | Uncommon |
|---|---|
|
|
Cause
Alpha-thalassemias are most commonly inherited in a Mendelian recessive manner. They are also associated with deletions of chromosome 16p.[2] Alpha thalassemia can also be acquired under rare circumstances.[12]
Pathophysiology
The mechanism sees that α thalassemias results in decreased alpha-globin production, therefore fewer alpha-globin chains are produced, resulting in an excess of β chains in adults and excess γ chains in newborns. The excess β chains form unstable tetramers called hemoglobin H or HbH of four beta chains. The excess γ chains form tetramers which are poor carriers of O2 since their affinity for O2 is too high, so it is not dissociated in the periphery. Homozygote α0 thalassaemias, where numerous γ4 but no α-globins occur at all (referred to as Hb Barts), often result in death soon after birth.[3][1][13]
Diagnosis
Diagnosis of alpha-thalassemia is primarily by laboratory evaluation and molecular diagnosis. Alpha-thalassemia can be mistaken for iron-deficiency anaemia on a full blood count or blood film, as both conditions have a microcytic anaemia. Serum iron and serum ferritin can be used to exclude iron-deficiency anaemia.[3]
Types
Two genetic loci exist for α globin, thus four alleles are in diploid cells. Two alleles are maternal and two alleles are paternal in origin. The severity of the α-thalassemias is correlated with the number of affected α-globin; alleles: the greater, the more severe will be the manifestations of the disease. When noting the genotype, an "α" indicates a functional alpha chain, and '-' a pathological one.[1][13]
| Alleles affected | Description | Genotype | |||||
|---|---|---|---|---|---|---|---|
| One | This is known as alpha thalassemia silent and with this type, the effect on hemoglobin synthesis is minimal. Three α-globin genes are enough to permit normal hemoglobin production, and no clinical symptoms present. It occurs due to a deletion or non-deletion mutation.[1] | - α/α α | |||||
| Two | 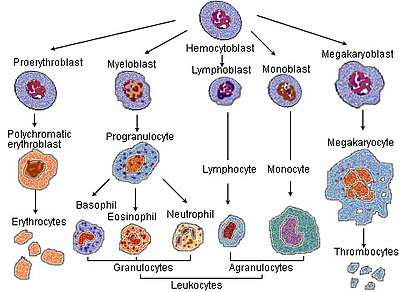 Haematopoiesis(production of blood cells) The condition is called alpha thalassemia trait; two α genes permit nearly normal production of red blood cells, but a mild microcytic hypochromic anemia is seen. The disease in this form can be mistaken for iron-deficiency anemia and treated inappropriately with iron.[3][1] Alpha-thalassemia trait can exist in two forms:[1]
|
- -/α α or - α/- α | |||||
| Three | This condition is called hemoglobin H disease; two unstable hemoglobins are present in the blood; hemoglobin Barts (tetrameric γ chains) and hemoglobin H (tetrameric β chains). Both of these unstable hemoglobins have a higher affinity for oxygen than normal hemoglobin.[14] A microcytic hypochromic anemia with target cells and Heinz bodies (precipitated HbH) on the peripheral blood smear can occur, as well as hepatosplenomegaly. The disease is noticed in childhood or in early adult life; anemia and hepatosplenomegaly are noted. | - -/- α | |||||
| Four | This is known as alpha thalassemia major; these fetuses are edematous, have little circulating hemoglobin, and the hemoglobin that is present is all tetrameric γ chains. When all four alleles are affected, the fetus likely will not survive gestation without in utero intervention; most infants with alpha-thalassemia major are stillborn with hydrops fetalis. Fetuses treated with intrauterine transfusions throughout pregnancy starting at an early gestational age can survive to birth with acceptable morbidity. After birth, the treatment options include bone marrow transplantation or continued chronic transfusions.[15] | - -/- - | |||||
| α α/α α = normal: 'α α' before the '/' represents one chromosome, and 'α α' after the '/', its homologous chromosome. | |||||||
Laboratory Diagnosis
Initial laboratory diagnosis should include a complete blood count and red blood cell indices.[8] As well, a peripheral blood smear should be carefully reviewed.[8]
Hemoglobin analysis is important for the diagnosis of alpha-thalassemia as it determines the types and percentages of types of hemoglobin present.[16] Several different methods of hemoglobin analysis exist, including hemoglobin electrophoresis, capillary electrophoresis and high-performance liquid chromatography.[16]
Molecular analysis of DNA sequences (DNA analysis) can be used for the confirmation of a diagnosis of alpha-thalassemia, particularly for the detection of alpha-thalassemia carriers (deletions or mutations in only one or two alpha-globin genes).[16]
Treatment
Treatment for alpha-thalassemia may include blood transfusions to maintain hemoglobin at a level that reduces symptoms of anemia. The decision to initiate transfusions depends on the clinical severity of the disease.[17] Splenectomy is a possible treatment option to increase total hemoglobin levels in cases of worsening anemia due to an overactive or enlarged spleen, or when transfusion therapy is not possible.[18] However, splenectomy is avoided when other options are available due to an increased risk of serious infections and thrombosis.[18]
Additionally, gallstones may be a problem that would require surgery. Secondary complications from febrile episode should be monitored, and most individuals live without any need for treatment.[1][4]
Additionally, stem cell transplantation should be considered as a treatment (and cure), which is best done in early age. Other options, such as gene therapy, are still being developed.[19]
A study by Kreger et al combining a retrospective review of three cases of alpha thalassemia major and a literature review of 17 cases found that in utero transfusion can lead to favorable outcomes. Successful hematopoietic cell transplantation was eventually carried out in four patients.[20]
Epidemiology
Worldwide distribution of inherited alpha-thalassemia corresponds to areas of malaria exposure, suggesting a protective role. Thus, alpha-thalassemia is common in sub-Saharan Africa, the Mediterranean Basin, and generally tropical (and subtropical) regions. The epidemiology of alpha-thalassemia in the US reflects this global distribution pattern. More specifically, HbH disease is seen in Southeast Asia and the Middle East, while Hb Bart hydrops fetalis is acknowledged in Southeast Asia only.[21] The data indicate that 15% of the Greek and Turkish Cypriots are carriers of beta-thalassaemia genes, while 10% of the population carry alpha-thalassaemia genes.[22]
See also
References
- Origa, Raffaella; Moi, Paolo; Galanello, Renzo; Cao, Antonio (1 January 1993). "Alpha-Thalassemia". GeneReviews. Retrieved 22 September 2016.update 2013
- BRS Pathology (4th ed.). Lippincott Williams & Wilkins medical. December 2009. p. 162. ISBN 978-1451115871.
- "Alpha Thalassemia Workup: Approach Considerations, Laboratory Studies, Hemoglobin Electrophoresis". emedicine.medscape.com. Retrieved 24 May 2016.
- "Complications and Treatment | Thalassemia | Blood Disorders | NCBDDD | CDC". www.cdc.gov. Retrieved 22 September 2016.
- Online Mendelian Inheritance in Man (OMIM) Hemoglobin—Alpha locus 1; HBA1 -141800
- Online Mendelian Inheritance in Man (OMIM) Hemoglobin—Alpha locus 2; HBA2 -141850
- Lanzkowsky's Manual Of Pediatric Hematology And Oncology 6th Edition ( 2016).
- "Alpha-thalassemia - Symptoms, diagnosis and treatment | BMJ Best Practice". bestpractice.bmj.com. Retrieved 17 November 2019.
- Reference, Genetics Home. "Alpha thalassemia". Genetics Home Reference. Retrieved 25 November 2019.
- Origa, Raffaella; Moi, Paolo (1993), Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Alpha-Thalassemia", GeneReviews®, University of Washington, Seattle, PMID 20301608, retrieved 25 November 2019
- "Assessment of anaemia - Aetiology | BMJ Best Practice". bestpractice.bmj.com. Retrieved 25 November 2019.
- Steensma DP, Gibbons RJ, Higgs DR (January 2005). "Acquired alpha-thalassemia in association with myelodysplastic syndrome and other hematologic malignancies". Blood. 105 (2): 443–52. doi:10.1182/blood-2004-07-2792. PMID 15358626.
- Galanello R, Cao A (February 2011). "Gene test review. Alpha-thalassemia". Genetics in Medicine. 13 (2): 83–8. doi:10.1097/GIM.0b013e3181fcb468. PMID 21381239.
- "Hemoglobin H disease". Orphanet. Retrieved 22 September 2016.
- Vichinsky EP (1 January 2009). "Alpha thalassemia major--new mutations, intrauterine management, and outcomes". Hematology. American Society of Hematology. Education Program. 2009 (1): 35–41. doi:10.1182/asheducation-2009.1.35. PMID 20008180.
- Viprakasit, Vip; Ekwattanakit, Supachai (1 April 2018). "Clinical Classification, Screening and Diagnosis for Thalassemia". Hematology/Oncology Clinics of North America. Thalassemia. 32 (2): 193–211. doi:10.1016/j.hoc.2017.11.006. ISSN 0889-8588. PMID 29458726.
- "UpToDate". www.uptodate.com. Retrieved 25 November 2019.
- Taher, Ali; Musallam, Khaled; Cappellini, Maria Domenica, eds. (2017). Guidelines for the management of non-transfusion-dependent thalassemia (NTDT) (2nd edition). Thalassemia International Foundation. pp. 24–32. Retrieved 5 November 2019.
- "Thalassaemia | Doctor | Patient". Patient. Retrieved 22 September 2016.
- Kreger EM, Singer ST, Witt RG, Sweeters N, Lianoglou B, Lal A, et al. (December 2016). "Favorable outcomes after in utero transfusion in fetuses with alpha thalassemia major: a case series and review of the literature". Prenatal Diagnosis. 36 (13): 1242–1249. doi:10.1002/pd.4966. PMID 27862048.
- Harteveld CL, Higgs DR (May 2010). "Alpha-thalassaemia". Orphanet Journal of Rare Diseases. 5 (1): 13. doi:10.1186/1750-1172-5-13. PMC 2887799. PMID 20507641.
- Haematology Made Easy. AuthorHouse. 2013-02-06. ISBN 9781477246511.page 246
Further reading
- Anie KA, Massaglia P (March 2014). "Psychological therapies for thalassaemia". The Cochrane Database of Systematic Reviews (3): CD002890. doi:10.1002/14651858.cd002890.pub2. PMID 24604627.
- Galanello R, Cao A (February 2011). "Gene test review. Alpha-thalassemia". Genetics in Medicine. 13 (2): 83–8. doi:10.1097/GIM.0b013e3181fcb468. PMID 21381239.
External links
- "What Are Thalassemias? - NHLBI, NIH". www.nhlbi.nih.gov. Retrieved 15 September 2016.
| Classification | |
|---|---|
| External resources |
|