Agar plate
An agar plate is a Petri dish that contains agar as a solid growth medium plus nutrients, used to culture microorganisms. Sometimes selective compounds are added to influence growth, such as antibiotics.[1]

| Uses | Microbiological culture Art |
|---|---|
| Related items | Petri dish Growth medium |
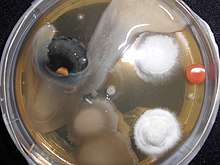
Individual microorganisms placed on the plate will grow into individual colonies, each a clone genetically identical to the individual ancestor organism (except for the low, unavoidable rate of mutation). Thus, the plate can be used either to estimate the concentration of organisms in a liquid culture or a suitable dilution of that culture using a colony counter, or to generate genetically pure cultures from a mixed culture of genetically different organisms.
Several methods are available to plate out cells. One technique is known as "streaking". In this technique, a drop of the culture on the end of a thin, sterile loop of wire, sometimes known as an inoculator, is streaked across the surface of the agar leaving organisms behind, a higher number at the beginning of the streak and a lower number at the end. At some point during a successful "streak", the number of organisms deposited will be such that distinct individual colonies will grow in that area which may be removed for further culturing, using another sterile loop.[1]
Another way of plating organisms, next to streaking, on agar plates is the spot analysis. This type of analysis is often used to check the viability of cells and performed with pinners (often also called froggers). A third used technique is the use of sterile glass beads to plate out cells. In this technique cells are grown in a liquid culture of which a small volume is pipetted on the agar plate and then spread out with the beads. Replica plating is another technique in order to plate out cells on agar plates. These four techniques are the most common, but others are also possible. It is crucial to work in a sterile manner in order to prevent contamination on the agar plates. Plating is thus often done in a laminar flow cabinet or on the working bench next to a bunsen burner.[2]
History
In 1881, Fanny Hesse, who was working as a technician for her husband Walther Hesse in the laboratory of Robert Koch, suggested agar as an effective setting agent, since it had been commonplace in jam making for some time.[3]
Types

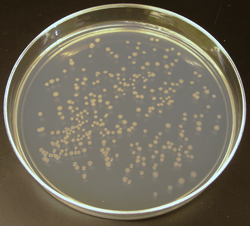
Like other growth media, the formulations of agar used in plates may be classified as either "defined" or "undefined"; a defined medium is synthesized from individual chemicals required by the organism so the exact molecular composition is known, whereas an undefined medium is made from natural products such as yeast extract, where the precise composition is unknown.[4]
Agar plates may be formulated as either permissive, with the intent of allowing the growth of whatever organisms are present, or restrictive or selective, with the intent of only allowing growth a particular subset of those organisms.[5] This may take the form of a nutritional requirement, for instance providing a particular compound such as lactose as the only source of carbon and thereby selecting only organisms which can metabolize that compound, or by including a particular antibiotic or other substance to select only organisms which are resistant to that substance. This correlates to some degree with defined and undefined media; undefined media, made from natural products and containing an unknown combination of very many organic molecules, is typically more permissive in terms of supplying the needs of a wider variety of organisms, while defined media can be precisely tailored to select organisms with specific properties.
Agar plates may also be indicator plates, in which the organisms are not selected on the basis of growth, but are instead distinguished by a color change in some colonies, typically caused by the action of an enzyme on some compound added to the medium.[6]
The plates are incubated for 12 hours up to several days depending on the test that is performed.
Some commonly used agar plate types are:
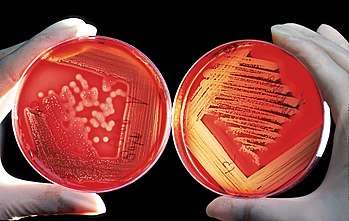
Blood agar

Blood agar plate
Blood agar plates (BAPs) contain mammalian blood (usually sheep or horse), typically at a concentration of 5–10%. BAPs are enriched, differential media used to isolate fastidious organisms and detect hemolytic activity. β-Hemolytic activity will show lysis and complete digestion of red blood cell contents surrounding a colony. Examples include Streptococcus haemolyticus. α-Hemolysis will only cause partial lysis of the red blood cells (the cell membrane is left intact) and will appear green or brown, due to the conversion of hemoglobin to methemoglobin. An example of this would be Streptococcus viridans. γ-Hemolysis (or nonhemolytic) is the term referring to a lack of hemolytic activity.[7] BAPs also contain meat extract, tryptone, sodium chloride, and agar.
Chocolate agar
Chocolate agar a type of blood agar plate in which the blood cells have been lysed by heating the cells to 56 °C. It is used for growing fastidious respiratory bacteria, such as Haemophilus influenzae. No chocolate is actually contained in the plate; it is named for the coloration only.
Horse blood agar
Horse blood agar is a type of blood-enriched microbiological culture media. As it is enriched, it allows the growth of certain fastidious bacteria, and allows indication of haemolytic activity in these bacterial cultures.
Thayer-Martin agar
Thayer-Martin agar is a chocolate agar designed to isolate Neisseria gonorrhoeae.
Thiosulfate-citrate-bile salts-sucrose agar
Thiosulfate-citrate-bile salts-sucrose agar enhances growth of Vibrio spp., including Vibrio cholerae.[8]
General bacterial media
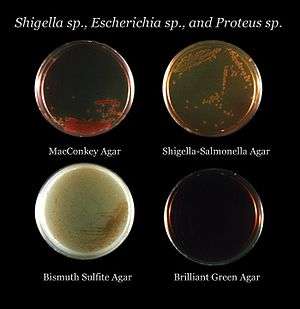
- Bile esculin agar is used for the isolation of Enterococcus and group D Streptococcus species
- CLED agar – cysteine, lactose, electrolyte-deficient agar is used to isolate and differentiate urinary tract bacteria, since it inhibits Proteus species swarming and can differentiate between lactose fermenters and nonfermenters.
- Granada medium is used to isolate and differentiate group B Streptococcus, Streptococcus agalactiae from clinical samples. It grows in Granada medium as red colonies and most of accompanying bacteria are inhibited.
- Hektoen enteric agar is designed to isolate and recover fecal bacteria of the family Enterobacteriaceae. It is particularly useful in isolating Salmonella and Shigella.
- Lysogeny broth
- MacConkey agar is a selective and differential medium used to differentiate between Gram-negative bacteria while inhibiting the growth of Gram-positive bacteria. The addition of bile salts and crystal violet to the agar inhibits the growth of most Gram-positive bacteria, making MacConkey agar selective. Lactose and neutral red are added to differentiate the lactose fermenters, which form pink colonies, from lactose nonfermenters that form clear colonies. An alternative medium, eosin methylene blue serves a similar purpose.
- Mannitol salt agar is also a selective and differential medium. The mannitol indicates organisms that ferment mannitol: mannitol fermentation produces lactic acid, lowering the pH and turning the plate yellow. The salt is to select for halophiles; organisms that cannot withstand a high salt content are unable to grow well.
- Mueller-Hinton agar contains beef infusion, peptone, and starch, and is used primarily for antibiotic susceptibility testing. It can be in a form of blood agar.

- Nutrient agar is usually used for growth of nonfastidious organisms and observation of pigment production. It is safe to use in school science laboratories because it does not selectively grow pathogenic bacteria.
- Önöz agar allows more rapid bacteriological diagnosis, as Salmonella and Shigella colonies can be clearly and reliably differentiated from other Enterobacteriaceae. The yields of Salmonella from stool samples obtained, when using this medium, are higher than those obtained with LEIFSON agar or Salmonella–Shigella agar.
- Phenylethyl alcohol agar selects for Staphylococcus species while inhibiting Gram-negative bacilli (e.g., Escherichia coli, Shigella, Proteus, etc.).
- R2A agar, a nonspecific medium, imitates water, so is used for water analysis.
- Tryptic (trypticase) soy agar (TSA) is a general-purpose medium produced by enzymatic digestion of soybean meal and casein. It is frequently the base medium of other agar types; for example, blood agar plates are made by enriching TSA plates with blood. TSA plates support growth of many semifastidious bacteria, including some species of Brucella, Corynebacterium, Listeria, Neisseria, and Vibrio.
- Xylose-lysine-deoxycholate agar is used for the culture of stool samples and contains two indicators. It is formulated to inhibit Gram-positive bacteria, while the growth of Gram-negative bacilli is encouraged. The colonies of lactose fermenters appear yellow. It is also used to culture possible Salmonella that may be present in a food sample. Most Salmonella colonies produce a black centre on it.
- Cetrimide agar is used for the selective isolation of the Gram-negative bacterium Pseudomonas aeruginosa.
- Tinsdale agar contains potassium tellurite, which can isolate Corynebacterium diphteriae.[8]
Fungal media

- Sabouraud agar is used to culture fungi and has a low pH that inhibits the growth of most bacteria; it also contains the antibiotic gentamicin to specifically inhibit the growth of Gram-negative bacteria.
- Hay infusion agar is specific for the culturing of slime moulds (which are not fungi).
- Potato dextrose agar is used to culture certain types of fungi.
- Malt extract agar has a high content of peptone and is acidic. It is essentially used in the isolation of fungal microorganisms.
Moss media
- Knop agar is used to axenically culture protonema and whole moss plants, such as those of Physcomitrella patens, a model organism.[9]
Yeast media
- YEPD media is often used as a general growth media for yeasts like Saccharomyces cerevisiae and Candida albicans
- Sporulation medium is medium used when spores have to be formed. It can also be used when working with fungi or bacteria depending on whether or not the strain is capable of forming spores.
_cells.jpg)
See also
- Microbial art
- Viral plaque
Different specific types of agar:
References
- Madigan M, Martinko J, eds. (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 0-13-144329-1.
- Sanders, Erin R. (11 May 2012). "Aseptic Laboratory Techniques: Plating Methods". Journal of Visualized Experiments (63). doi:10.3791/3064. PMC 4846335. Archived from the original on 14 November 2017. Retrieved 3 May 2018.
- "History of the agar plate". Laboratory News. Archived from the original on 11 February 2010. Retrieved 2010-02-22.
- Baron S; et al., eds. (1996). Baron's Medical Microbiology (4th ed.). University of Texas Medical Branch. (via NCBI Bookshelf) ISBN 0-9631172-1-1.
- Ryan KJ; Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
- "Indicator Plates". Retrieved 12 July 2018.
- "Blood Agar Plates and Hemolysis Protocols". Archived from the original on 2012-02-02. Retrieved 2014-10-28.
- Fisher, Bruce; Harvey, Richard P.; Champe, Pamela C. Lippincott's Illustrated Reviews: Microbiology (Lippincott's Illustrated Reviews Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-8215-5.
- Ralf Reski and Wolfgang O. Abel (1985): Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 165, 354–358.
External links
| The Wikibook School Science has a page on the topic of: Agar plate |
| Wikimedia Commons has media related to Agar plates. |