Afamelanotide
Afamelanotide (melanotan I, brand name Scenesse)[2] is a synthetic peptide and analogue of α-melanocyte stimulating hormone used to prevent skin damage from the sun in people with erythropoietic protoporphyria in Europe since January 2015, and the United States since October 2019. As a medicine sold under the brand name "Scenesse", it is administered in subcutaneous implant form; the implant lasts for two months.
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌæfəmɛˈlænoʊtaɪd/ ( |
| Trade names | Scenesse |
| Other names | [Nle4,D-Phe7]α-MSH; NDP-α-MSH; NDP-MSH; Melanotan; Melanotan-1; Melanotan I; EPT1647; CUV1647; |
| AHFS/Drugs.com | UK Drug Information |
| License data |
|
| Routes of administration | S.C.; I.M.; I.V.; subcutaneous implant; intranasal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 30 minutes[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C78H111N21O19 |
| Molar mass | 1646.845 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It is under development in other skin disorders in several jurisdictions. It causes skin to turn darker by causing the skin to make more melanin.
It was discovered at University of Arizona and initially developed there as a sunless tanning agent; the Australian company Clinuvel conducted further clinical trials in that and other indications, and brought the drug to market in Europe and the United States.
Unlicensed and untested powders sold as "melanotan" are found on the Internet marketed for tanning and other purposes, and multiple regulatory bodies have warned consumers that the peptides may be unsafe and ineffective.
Medical use
Afamelanotide is used in Europe to prevent phototoxicity in adults with erythropoietic protoporphyria (EPP).[1][3] It is an implant that is injected and placed under the skin; an implant lasts two months.[1]
People who have severe liver disease, liver impairment, or kidney impairment, should not use this drug. Pregnant women should not take it, and women who are active sexually should use contraception while they are taking it. It is not known if afamelanotide is secreted in breast milk.[1]
Adverse effects
Very common (up to 10% of people) adverse effects in people with EPP include headache and nausea. Common (between 1% and 10%) adverse effects include back pain, upper respiratory tract infections, decreased appetite, migraine, dizziness, weakness, fatigue, lethargy, sleepiness, feeling hot, stomach pain, diarrhea, vomiting, flushing and red skin, development of warts, spots, and freckles, itchy skin, and reactions at the injection site. There are many uncommon (less than 1%) adverse effects.[1]
Pharmacology
Afamelanotide is thought to cause skin to darken by binding to the melanocortin 1 receptor which in turn drives melanogenesis.[1]
Afamelanotide has a half-life of 30 minutes. After the implant is injected, most of the drug is released within the first two days, with 90% released by the fifth day. By the tenth day no drug is detectable in plasma.[1]
Its metabolites, distribution, metabolism and excretion were not understood as of 2017.[1]
Chemistry
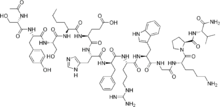
The amino acid sequence is Ac-Ser-Tyr-Ser-Nle-Glu-His-D-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2, and it is additionally known as [Nle4,D-Phe7]-α-MSH, which is sometimes abbreviated as NDP-MSH or NDP-α-MSH. Afamelanotide is the International Nonproprietary Name.[4]
History
The role of α-MSH in promoting melanin diffusion has been known since the 1960s.[5] In the 1980s, scientists at University of Arizona began attempting to develop α-MSH and analogs as potential sunless tanning agents, and synthesized and tested several analogs, including melanotan-I.[6]
To pursue the tanning agent, melanotan-I was licensed by Competitive Technologies, a technology transfer company operating on behalf of University of Arizona, to an Australian startup called Epitan,[7][6] which changed its name to Clinuvel in 2006.[8]
Early clinical trials showed that the peptide had to be injected about ten times a day due to its short half-life, so the company collaborated with Southern Research in the US to develop a depot formulation that would be injected under the skin, and release the peptide slowly. This was done by 2004.[7]
As of 2010, afamelanotide was in Phase III trials for erythropoietic protoporphyria and polymorphous light eruption, and was in Phase II trials for actinic keratosis and squamous cell carcinoma, and had been trialled in phototoxicity associated with systemic photodynamic therapy and solar urticaria.[9] Clinuvel had also obtained orphan drug status for afamelanotide in the US and the EU by that time.[9]
In May 2010, the Italian Medicines Agency (AIFA, or Agenzia Italiana del Farmaco) approved afamelanotide as a treatment for erythropoietic protoporphyria.[10]
In January 2015, afamelanotide was approved by the European Medicines Agency (EMA) in Europe for the treatment of phototoxicity in people with EPP.[1]
In October 2019, afamelanotide was approved by the US Food and Drug Administration (FDA) as a medicine to reduce pain caused by light exposure (particularly sunlight) as experienced by sufferers of erythropoietic protoporphyria.[11]
Society and culture
Counterfeits
A number of products are sold online and in gyms and beauty salons as "melanotan" or "melanotan-1" which discuss afamelanotide in their marketing.[12][13] [14]
Without a prescription the products as drugs are not legally sold in any jurisdiction and are potentially dangerous.[15][16][17][18]
Starting in 2007, health agencies in various counties began issuing warnings against their use.[19][20][21][22] [23][24]
See also
- BMS-470,539
- Bremelanotide
- Melanotan II
- Methoxsalen
- Modimelanotide
- Setmelanotide
References
- "Scenesse: Summary of Product Characteristics" (PDF). EMA. 27 January 2016. Retrieved 6 April 2017. For updates see EMA Index page
- "Afamelanotide". AdisInsight. Retrieved 6 April 2017.
- "Scenesse". European Medicines Agency. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
- "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). World Health Organization. 2009. Retrieved 2 March 2009.
- Baker, BI (31 May 1993). "The role of melanin-concentrating hormone in color change". Annals of the New York Academy of Sciences. 680: 279–89. doi:10.1111/j.1749-6632.1993.tb19690.x. PMID 8390154.
- Hadley, ME; Dorr, RT (April 2006). "Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization". Peptides. 27 (4): 921–30. doi:10.1016/j.peptides.2005.01.029. PMID 16412534.
- "EpiTan focuses on Melanotan, a potential blockbuster". The Pharma Letter. 1 November 2004.
- "Epitan changes name to Clinuvel, announces new clinical program". LabOnline. 27 February 2006.
- Dean, Tim (3 May 2010). "Biotechnology profile: Bright future for Clinuvel (ASX:CUV)". Australian Life Scientist. Archived from the original on 6 April 2017.
- "Gazzetta Ufficiale: Sommario". Agenzia Nazionale Stampa Associata. 2010. Retrieved 17 May 2010.
- "FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder" (Press release). 8 October 2019. Archived from the original on 8 October 2019.
- "Believe It Or Not 'Tanorexia' A Very Real Problem". WCBS-TV, CBS. 20 May 2009. Archived from the original on 21 May 2009. Retrieved 23 July 2009.
- "Fools Gold". Cosmopolitan (Australia). 14 June 2009. Retrieved 25 July 2009.
- Madrigal, Alexis (29 January 2009). "Suntan Drug Greenlighted for Trials". Wired. Archived from the original on 5 May 2009. Retrieved 11 April 2009.
- "Tanning drug a health risk". Herald Sun. 31 October 2009. Retrieved 31 October 2009.
- Ewan A Langan; Z. Nie; Lesley E Rhodes (June 2010). "Melanotropic peptides: More than just "Barbie drugs" and "sun tan jabs?"". British Journal of Dermatology. 163 (3): 451–5. doi:10.1111/j.1365-2133.2010.09891.x. PMID 20545686.
- Ewan A Langan; Denise Ramlogan; Lynne A Jamieson; Lesley E Rhodes (January 2009). "Change in moles linked to use of unlicensed "sun tan jab"". BMJ. 338: b277. doi:10.1136/bmj.b277. PMID 19174439.
- "Risky tan jab warnings 'ignored'". BBC. 18 February 2009. Archived from the original on 21 February 2009. Retrieved 4 March 2009.
- "Warning against the product Melanotan". Danish Medicines Agency. 2008. Retrieved 11 August 2008.
- ""Tan jab" is an unlicensed medicine and may not be safe" (Press release). Medicines and Healthcare products Regulatory Agency (MHRA). 2008. Archived from the original on 5 December 2014. Retrieved 17 November 2008.
- "US Lab Research Inc Warning letter". U.S. Food and Drug Administration (FDA). 29 January 2009. Archived from the original on 10 July 2009. Retrieved 23 July 2009.
- "Melanotan Powder for Injection". Notice Information: – Warning – 27 February 2009. Irish Medicines Board. 2009. Retrieved 2 February 2009.
- "Legemiddelverket advarer mot bruk av Melanotan". Norwegian Medicines Agency. 13 December 2007. Archived from the original on 17 April 2009. Retrieved 11 March 2009.
- "Melanotan – farlig og ulovlig brunfarge". Norwegian Medicines Agency. 23 January 2009. Archived from the original on 17 April 2009. Retrieved 11 March 2009.
External links
- "Afamelanotide". Drug Information Portal. U.S. National Library of Medicine.