Adrenal cortex
Situated along the perimeter of the adrenal gland, the adrenal cortex mediates the stress response through the production of mineralocorticoids and glucocorticoids, such as aldosterone and cortisol, respectively. It is also a secondary site of androgen synthesis.[2]
| Adrenal cortex | |
|---|---|
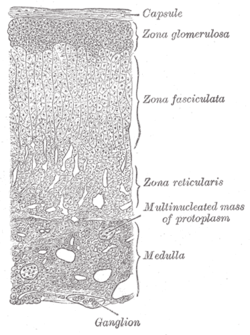 Layers of cortex. | |
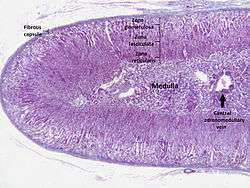 The adrenal cortex | |
| Details | |
| Precursor | mesoderm[1] |
| Identifiers | |
| Latin | cortex glandulae suprarenalis |
| MeSH | D000302 |
| TA | A11.5.00.007 |
| FMA | 15632 |
| Anatomical terminology | |
Layers
The adrenal cortex comprises three main zones, or layers that are regulated by distinct hormones as noted below. This anatomic zonation can be appreciated at the microscopic level, where each zone can be recognized and distinguished from one another based on structural and anatomic characteristics.[3]
- Zona glomerulosa
- The outermost layer, the zona glomerulosa is the main site for the production of aldosterone, a mineralocorticoid. The synthesis and secretion of aldosterone are mainly regulated by the renin–angiotensin–aldosterone system. The zona glomerulosa cells express a specific enzyme aldosterone synthase (also known as CYP11B2).[4][5] Aldosterone is largely responsible for the long-term regulation of blood pressure.[6] Aldosterone's effects are on the distal convoluted tubule and collecting duct of the kidney where it causes increased reabsorption of sodium and increased excretion of both potassium (by principal cells) and hydrogen ions (by intercalated cells of the collecting duct).[6] Sodium retention is also a response of the distal colon, and sweat glands to aldosterone receptor stimulation. Although sustained production of aldosterone requires persistent calcium entry through low-voltage activated Ca2+ channels, isolated zona glomerulosa cells are considered nonexcitable, with recorded membrane voltages that are too hyperpolarized to permit Ca2+ channels entry.[7]
- The secretion of aldosterone is also stimulated by ACTH.[8]
- The expression of neuron-specific proteins in the zona glomerulosa cells of human adrenocortical tissues has been predicted and reported by several authors[9][10][11] and it was suggested that the expression of proteins like the neuronal cell adhesion molecule (NCAM) in the cells of the zona glomerulosa reflects the regenerative feature of these cells, which would lose NCAM immunoreactivity after moving to the zona fasciculata.[9][12] However, together with other data on neuroendocrine properties of zona glomerulosa cells, NCAM expression may reflect a neuroendocrine differentiation of these cells.[9]
- Zona fasciculata
- Situated between the glomerulosa and reticularis, the cells of the zona fasciculata synthesize and secrete glucocorticoids, such as 11-deoxycorticosterone, corticosterone, and cortisol in humans. The major hormone that stimulates cortisol secretion in humans is the adrenocorticotropic hormone (ACTH) that is released from the anterior pituitary.[8]. It has been shown that the steroidogenic capacity of the zona fasciculata increases during illness in infants.[8]
- Zona reticularis
- The inner most cortical layer, the zona reticularis produces androgens, mainly dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), and androstenedione (the precursor to testosterone) in humans. The secretion of DHEAS is also stimulated by ACTH.[8]
Hormone synthesis
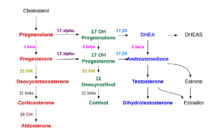
The precursor of steroids synthesized in the adrenal cortex is cholesterol that is stored in vesicles. Cholesterol can be synthesized de novo in the adrenal cortex. Yet, the major source of cholesterol appears to be cholesterol that is taken up with circulating lipoproteins. [13]
The steps up to this point occur in many steroid-producing tissues. Subsequent steps to generate aldosterone and cortisol, however, primarily occur in the adrenal cortex:
- Progesterone → (hydroxylation at C21) → 11-Deoxycorticosterone → (two further hydroxylations at C11 and C18) → Aldosterone
- Progesterone → (hydroxylation at C17) → 17-alpha-hydroxyprogesterone → (hydroxylation at C21) → 11-Deoxycortisol → (hydroxylation at C11) → Cortisol
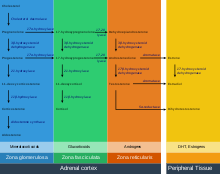
Production
The adrenal cortex produces a number of different corticosteroid hormones.
Mineralocorticoids
The primary mineralocorticoid, aldosterone, is produced in the adrenocortical zona glomerulosa by the action of the enzyme aldosterone synthase (also known as CYP11B2).[4][5] Aldosterone is largely responsible for the long-term regulation of blood pressure.[6] Aldosterone effects on the distal convoluted tubule and collecting duct of the kidney where it causes increased reabsorption of sodium and increased excretion of both potassium (by principal cells) and hydrogen ions (by intercalated cells of the collecting duct).[6] Sodium retention is also a response of the distal colon, and sweat glands to aldosterone receptor stimulation. Although sustained production of aldosterone requires persistent calcium entry through low-voltage activated Ca2+ channels, isolated zona glomerulosa cells are considered nonexcitable, with recorded membrane voltages that are too hyperpolarized to permit Ca2+ channels entry.[7]
Glucocorticoids
Glucocorticoids are produced in the zona fasciculata. The primary glucocorticoid released by the adrenal gland is cortisol in humans and corticosterone in many other animals. Its secretion is regulated by the hormone ACTH from the anterior pituitary.
Androgens
They are produced in the zona reticularis. The most important androgens include:
- Testosterone: a hormone with a wide variety of effects, ranging from enhancing muscle mass and stimulation of cell growth to the development of the secondary sex characteristics.
- Dihydrotestosterone (DHT): a metabolite of testosterone, and a more potent androgen than testosterone in that it binds more strongly to androgen receptors.
- Androstenedione (Andro): an androgenic steroid produced by the testes, adrenal cortex, and ovaries. While androstenediones are converted metabolically to testosterone and other androgens, they are also the parent structure of estrone.
- Dehydroepiandrosterone (DHEA): It is the primary precursor of natural estrogens. DHEA is also called dehydroisoandrosterone or dehydroandrosterone. The reticularis also produces DHEA-sulfate due to the actions of a sulfotransferase, SULT2A1.[14]
Pathology
See also
References
- "Embryology of the adrenal gland". Retrieved 2007-12-11.
- Anatomy Atlases - Microscopic Anatomy, plate 15.292 – "Adrenal Gland"
- Whitehead, Saffron A.; Nussey, Stephen (2001). Endocrinology: an integrated approach. Oxford: BIOS. p. 122. ISBN 978-1-85996-252-7.
- Curnow KM, Tusie-Luna MT, Pascoe L, et al. (October 1991). "The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex". Mol. Endocrinol. 5 (10): 1513–22. doi:10.1210/mend-5-10-1513. PMID 1775135.
- Zhou M, Gomez-Sanchez CE (July 1993). "Cloning and expression of a rat cytochrome P-450 11 beta-hydroxylase/aldosterone synthase (CYP11B2) cDNA variant". Biochem. Biophys. Res. Commun. 194 (1): 112–7. doi:10.1006/bbrc.1993.1792. PMID 8333830.
- Marieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14
- Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ (June 2012). "Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators". J. Clin. Invest. 122 (6): 2046–53. doi:10.1172/JCI61996. PMC 3966877. PMID 22546854.
- Hanukoglu A, Fried D, Nakash I, Hanukoglu I (Nov 1995). "Selective increases in adrenal steroidogenic capacity during acute respiratory disease in infants". Eur J Endocrinol. 133 (5): 552–6. doi:10.1530/eje.0.1330552. PMID 7581984.
- Ehrhart-Bornstein M, Hilbers U (1998). "Neuroendocrine properties of adrenocortical cells". Horm. Metab. Res. 30 (6–7): 436–9. doi:10.1055/s-2007-978911. PMID 9694576.
- Lefebvre H, Cartier D, Duparc C, et al. (March 2002). "Characterization of serotonin(4) receptors in adrenocortical aldosterone-producing adenomas: in vivo and in vitro studies". J. Clin. Endocrinol. Metab. 87 (3): 1211–6. doi:10.1210/jcem.87.3.8327. PMID 11889190.
- Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE (October 2007). "G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism". J. Endocrinol. 195 (1): 39–48. doi:10.1677/JOE-07-0037. PMID 17911395.
- Haidan A, Bornstein SR, Glasow A, Uhlmann K, Lübke C, Ehrhart-Bornstein M (February 1998). "Basal steroidogenic activity of adrenocortical cells is increased 10-fold by coculture with chromaffin cells". Endocrinology. 139 (2): 772–80. doi:10.1210/endo.139.2.5740. PMID 9449652.
- London E, Wassif CA, Horvath A, Tatsi C, Angelousi A, Karageorgiadis AS, Porter FD, Stratakis CA (2015). "Cholesterol Biosynthesis and Trafficking in Cortisol-Producing Lesions of the Adrenal Cortex". J Clin Endocrinol Metab. 100 (10): 3660–7. doi:10.1210/jc.2015-2212. PMC 4596036. PMID 26204136.CS1 maint: multiple names: authors list (link)
- Rainey WE, Nakamura Y (February 2008). "Regulation of the adrenal androgen biosynthesis". J. Steroid Biochem. Mol. Biol. 108 (3–5): 281–6. doi:10.1016/j.jsbmb.2007.09.015. PMC 2699571. PMID 17945481.
External links
- Anatomy photo:40:04-0203 at the SUNY Downstate Medical Center – "Posterior Abdominal Wall: Blood Supply to the Suprarenal Glands"
- MedicalMnemonics.com: 180 2201 412
- Histology image: 14502loa – Histology Learning System at Boston University