Adavosertib
Adavosertib (development codes AZD1775, MK-1775) is an experimental anti-cancer drug candidate. It is a small molecule inhibitor of the tyrosine kinase WEE1 with potential antineoplastic sensitizing activity.[1] It is being developed by AstraZeneca.[2]It is being investigated as a treatment for pancreatic cancer with phase 1 trial.[3][4] University of Michigan researchers are as of 2019 planning a phase 2 study.[5]
 | |
| Names | |
|---|---|
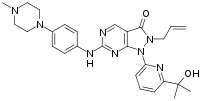
| IUPAC name
1-[6-(2-Hydroxypropan-2-yl)pyridin-2-yl]-6-[4-(4-methylpiperazin-1-yl)anilino]-2-prop-2-enylpyrazolo[3,4-d]pyrimidin-3-one | |
| Other names
AZD1775; MK-1775 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.205.373 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C27H32N8O2 |
| Molar mass | 500.607 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- "NCI Drug Dictionary". National Cancer Institute. 2 February 2011. Retrieved 17 August 2019.
- "AZD1775 | AstraZeneca Open Innovation". openinnovation.astrazeneca.com. Retrieved 17 August 2019.
- "Early Activity in Pancreatic Cancer With New Drug". www.medpagetoday.com. 16 August 2019. Retrieved 17 August 2019.
- Cuneo, KC; Morgan, MA; Sahai, V; Schipper, MJ; Parsels, LA; Parsels, JD; Devasia, T; Al-Hawaray, M; Cho, CS; Nathan, H; Maybaum, J; Zalupski, MM; Lawrence, TS (9 August 2019). "Dose Escalation Trial of the Wee1 Inhibitor Adavosertib (AZD1775) in Combination With Gemcitabine and Radiation for Patients With Locally Advanced Pancreatic Cancer". Journal of Clinical Oncology. 37 (29): 2643–2650. doi:10.1200/JCO.19.00730. PMID 31398082.
- "AstraZeneca drug heads to phase 2 in pancreatic cancer after small trial extends survival". FierceBiotech. Retrieved 17 August 2019.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.